| Preoperative Evaluation | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | RCT | NRSI | Expanded | Standard | GRADE* | Effect | Estimate (95% CI) |
| N (Total) | N (Total) | ||||||
| Delirium incidence | 6 | 189 (662) | 253 (703) | RR | 0.77 (0.60–0.99) | ||
| 6 | 135 (1,126) | 176 (984) | RR | 0.64 (0.47–0.86) | |||
| Hip fracture | 4 | 168 (450) | 212 (479) | RR | 0.84 (0.70–0.99) | ||
| Other | 2 | 21 (212) | 41 (224) | RR | 0.54 (0.33–0.89) | ||
| Neurocognitive disorder <30 days | 1 | 13 (96) | 16 (84) | RR | 0.71 (0.36-1.39) | ||
| Neurocognitive disorder 30 days to 1 yr | none | none | |||||
| Physical function | 5 | (563) | (576) | SMD | 0.09 (-0.16 to 0.31) | ||
| 4 | (407) | (370) | SMD | 0.02 (-0.33 to 0.37) | |||
| Complications† | 4 | 9 | see below | ||||
| Patient satisfaction | 1 | 32 (32) | 29 (30) | RD/100 | 3.3 (-5.3 to 12.0)‡ | ||
| Length of stay (days) | 8 | (968) | (1,001) | MD | 0.0 (-1.7 to 1.7) | ||
| 14 | (1,972) | (1,889) | MD | -1.1 (-2.0 to -0.1) | |||
| Discharge to institution | 4 | 252 (419) | 271 (424) | RR | 0.98 (0.76-1.27) | ||
| 6 | 345 (855) | 336 (668) | RR | 0.82 (0.63–1.07) | |||
| Mortality (in-hospital or 30-day) | 4 | 19 (498) | 19 (526) | RR | 1.02 (0.30–3.53) | ||
| 5 | 19 (561) | 19 (589) | RD/1000 | 0.2 (-31.6 to 32.0) | |||
| 15 | 110 (5,746) | 205 (7,177) | RR | 0.66 (0.51–0.85) | |||
| 16 | 110 (5,846) | 205 (7,202) | RD/1000 | -7.5 (-14.1 to -0.8) | |||
| Mortality (1-year) | 2 | 42 (215) | 57 (226) | RR | 0.78 (0.54–1.10) | ||
| RD/1000 | -57.2 (-134.7 to 20.3) | ||||||
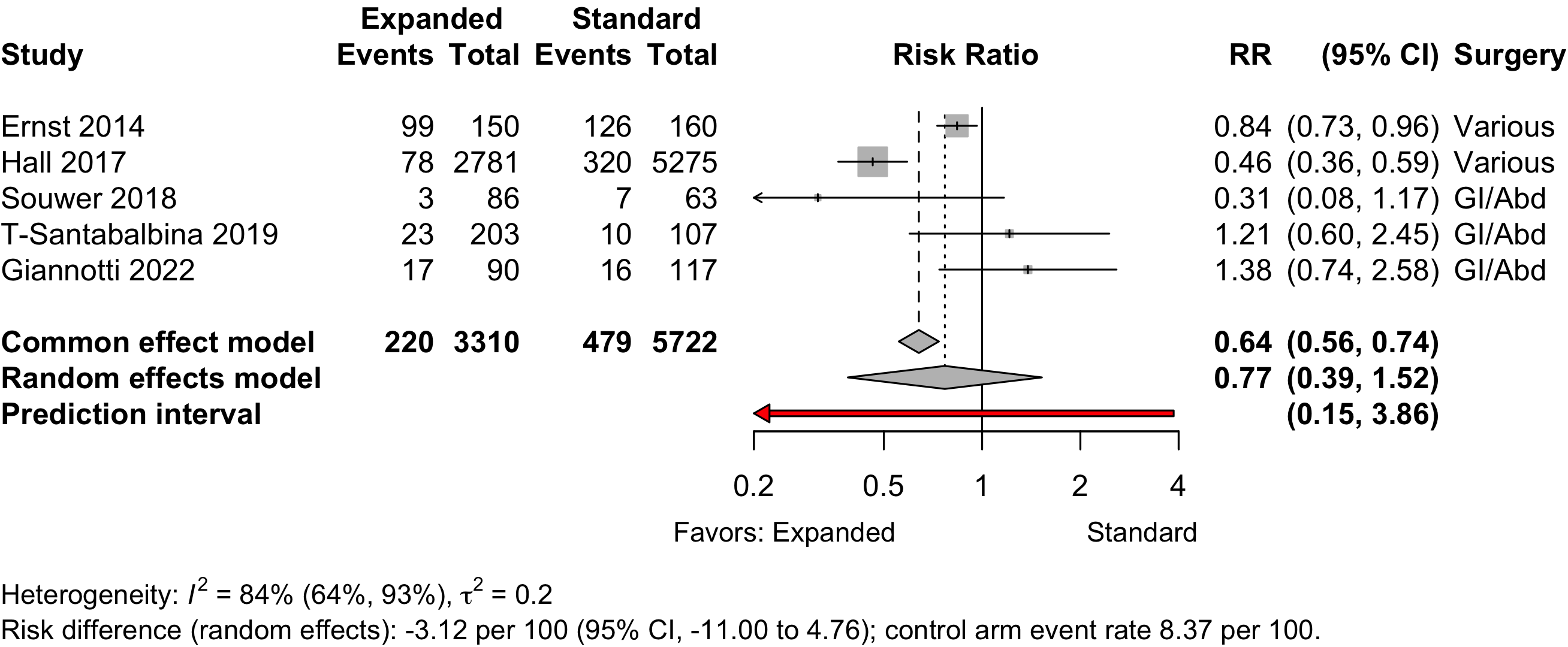
| 5 | 220 (3,310) | 479 (5,722) | RR | 0.77 (0.39–1.52) | |||
| RD/1000 | -31.5 (-113.1 to 50.1) | ||||||
| RCT: randomized clinical trial; NRSI: nonrandomized studies of interventions (non-randomized trial, before-after and cohort studies); GRADE: Grades of Recommendation, Assessment, Development, and Evaluation; RR: risk ratio; SMD: standardized mean difference; RD: risk difference; MD: mean difference. | |||||||
| * Very low: ⨁◯◯◯; Low: ⨁⨁◯◯; Moderate: ⨁⨁⨁◯; High: ⨁⨁⨁⨁. | |||||||
| † Cardiovascular, pulmonary, and renal. | |||||||
| ‡ High versus lower satisfaction. | |||||||
Expanded Preoperative Evaluation
Key Question
Among older patients anticipating surgery and anesthesia, does expanded preoperative evaluation (e.g., for frailty, cognitive impairment, functional status, or psychosocial issues) lead to improved postoperative outcomes?
Balance Tables
| Preoperative Evaluation | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | RCT | NRSI | Expanded | Standard | GRADE* | Effect | Estimate (95% CI) |
| N (Total) | N (Total) | ||||||
| Myocardial infarction | 1 | 4 | 9 (811) | 12 (714) | RR | 0.58 (0.22–1.53) | |
| RD/1000 | -2.7 (-17.7 to 12.3) | ||||||
| Cardiac arrest | 1 | 1 | 2 (268) | 7 (234) | RR | 0.28 (0.06–1.37) | |
| RD/1000 | 17.5 (-47.2 to 12.3) | ||||||
| Acute kidney injury | 2 | 4 | 85 (808) | 104 (805) | RR | 0.83 (0.60–1.16) | |
| RD/1000 | 3.4 (-26.8 to 33.7) | ||||||
| Pneumonia | 2 | 7 | 78 (1,261) | 94 (1,198) | RR | 0.82 (0.51–1.32) | |
| RD/1000 | -2.8 (-20.4 to 14.8) | ||||||
| Respiratory failure | 1 | 16 (183) | 21 (143) | RD/1000 | -5.9% (-13.0 to 1.2) | ||
| Pulmonary embolism | 4 | 6 (468) | 5 (354) | RR | 0.90 (0.26–3.08) | ||
| RD/1000 | 1.2 (-14.6 to 16.9) | ||||||
| RCT: randomized clinical trial; GRADE: Grades of Recommendation, Assessment, Development, and Evaluation; RR: risk ratio; RD: risk difference. | |||||||
| * Very low: ⨁◯◯◯; Low: ⨁⨁◯◯; Moderate: ⨁⨁⨁◯; High: ⨁⨁⨁⨁. | |||||||
Outcomes Reported
Table 2. Publications reporting dichotomous or count outcomes (not necessarily unique studies).
| Outcome | RCT, N = 11 | NR Trial, N = 1 | Quasi-exp, N = 18 | Prosp Coh, N = 1 | Retro Coh, N = 2 |
|---|---|---|---|---|---|
| ADL | 1 (9.1%) | — | — | — | — |
| Complications | 5 (45%) | — | 12 (67%) | 1 (100%) | 2 (100%) |
| DNCR/POCD | — | — | 1 (5.6%) | — | — |
| Delirium | 6 (55%) | — | 7 (39%) | — | 2 (100%) |
| Delirium duration | — | — | — | — | — |
| Discharge location | 4 (36%) | 1 (100%) | 4 (22%) | — | 1 (50%) |
| Mortality | 7 (64%) | 1 (100%) | 12 (67%) | 1 (100%) | 2 (100%) |
| Opioid use | — | — | — | — | — |
| Pain | 1 (9.1%) | — | 1 (5.6%) | — | — |
| QoR | — | — | — | — | — |
| Readmission | 5 (45%) | — | 8 (44%) | — | 2 (100%) |
| Satisfaction | — | — | 1 (5.6%) | — | — |
| ADL: activities of daily living; NCR: neurocognitive recovery; POCD: postoperative neurocognitive disorder; QoR: quality of recovery; RCT: randomized clinical trial; NR Trial: non-randomized trial; Quasi-exp: before-after or time series; Prosp Coh: prospective cohort; Retro Coh: retrospective cohort. | |||||
Table 3. Publications reporting continuous outcomes (not necessarily unique studies).
| Outcome | RCT, N = 11 | NR Trial, N = 1 | Quasi-exp, N = 18 | Prosp Coh, N = 1 | Retro Coh, N = 2 |
|---|---|---|---|---|---|
| Delirium duration | 2 (18%) | — | — | — | — |
| Length of stay | 10 (91%) | 1 (100%) | 11 (61%) | 1 (100%) | 2 (100%) |
| Opioid use | — | — | — | — | — |
| RCT: randomized clinical trial; NR Trial: non-randomized trial; Quasi-exp: before-after or time series; Prosp Coh: prospective cohort; Retro Coh: retrospective cohort. | |||||
Table 4. Publications reporting likert or ordinal outcomes (not necessarily unique studies).
| Outcome | RCT, N = 11 | NR Trial, N = 1 | Quasi-exp, N = 18 | Prosp Coh, N = 1 | Retro Coh, N = 2 |
|---|---|---|---|---|---|
| ADL | 6 (55%) | 1 (100%) | 3 (17%) | — | — |
| DNCR/POCD | 3 (27%) | — | — | — | — |
| Delirium | 1 (9.1%) | — | — | — | — |
| Complications | — | — | — | — | — |
| Pain | — | — | — | — | — |
| Quality of life | 1 (9.1%) | — | 2 (11%) | — | — |
| QoR | — | — | — | — | — |
| Satisfaction | — | — | — | — | — |
| ADL: activities of daily living; NCR: neurocognitive recovery; POCD: postoperative neurocognitive disorder; QoR: quality of recovery; RCT: randomized clinical trial; NR Trial: non-randomized trial; Quasi-exp: before-after or time series; Prosp Coh: prospective cohort; Retro Coh: retrospective cohort. | |||||
Included Studies
See Appendix for detailed summary study and patient characteristics including primary outcomes.
Table 5. Number of studies by design.
| Design | Studies |
|---|---|
| Randomized Clinical Trial | 9 |
| Nonrandomized Trial | 1 |
| Before-After/Time Series | 18 |
| Prospective Cohort | 1 |
| Retrospective Cohort | 2 |
| Total | 31 |
| Studies with multiple publications counted once (applies to 2 studies with 2 publications). | |
Design, centers, country, and surgery
Table 6. Study design, enrollment, centers, country, and surgery (see References for citations).
| ID | Study | Centers | Enrolled | Countrya | Surgery |
|---|---|---|---|---|---|
| Randomized Clinical Trial | |||||
| 741 | 1 | 126 | USA | HipFx | |
| 16608 | 1 | 397 | Norway | HipFx | |
| 17156 | 1 | 159 | Taiwana | HipFx | |
| 17155 | 1 | 162 | Taiwana | HipFx | |
| 16628 | 1 | 321 | Spain | HipFx | |
| 1201 | 1 | 329 | Norway | HipFx | |
| 16739 | 1 | 155 | Chinaa | HipFx | |
| 555 | 2 | 122 | Norway | GI/Abd | |
| 760 | 3 | 297 | Netherlands | Variousb | |
| 1263 | 3 | 297 | Netherlands | Variousb | |
| 16605 | 1 | 201 | UK | Variousb | |
| Nonrandomized Trial | |||||
| 17087 | 1 | 171 | Belgium | HipFx | |
| Before-After/Time Series | |||||
| 16544 | 1 | 276 | Sweden | HipFx | |
| 16629 | 1 | 1056 | Netherlands | HipFx | |
| 19171 | 1 | 108 | UK | Other Ortho | |
| 16601 | 2 | 266 | Sweden | Other Ortho | |
| 13133 | 1 | 181 | Italy | Other Ortho | |
| 16846 | 1 | 207 | Italy | GI/Abd | |
| 149 | 1 | 443 | Netherlands | GI/Abd | |
| 6017 | 1 | 149 | Netherlands | GI/Abd | |
| 18807 | 1 | 83 | Switzerland | GI/Abd | |
| 18797 | 1 | 281 | Netherlands | Cardiac | |
| 16539 | 1 | 241 | Netherlands | Variousb | |
| 2736 | 1 | 310 | USA | Variousb | |
| 1313 | 1 | 9153 | USA | Variousb | |
| 11264 | 1 | 492 | USA | Variousb | |
| 16591 | 1 | 326 | USA | Variousb | |
| 1294 | 1 | 62 | USA | Variousb | |
| 2108 | 1 | 242 | UK | Urol | |
| 1189 | 1 | 125 | USA | Neuro | |
| Prospective Cohort | |||||
| 17128 | 1 | 466 | USA | Variousb | |
| Retrospective Cohort | |||||
| 16623 | 1 | 310 | Spain | GI/Abd | |
| 19377 | 1 | 228 | France | Cardiac | |
| GI: gastrointestinal; Ortho: orthopedic; Neuro: neurological; Oralmax: oral maxillofacial; Vasc: vascular. | |||||
| a Non very-high Human Development Index country. | |||||
| b Described as various or more than 4 different types of surgery. | |||||
Country Summary
Table 7. Summary of studies by country where conducted.
| N = 31a | |
|---|---|
| Country | |
| USA | 8 (26%)b |
| Netherlands | 6 (19%)b |
| Norway | 3 (9.7%) |
| UK | 3 (9.7%) |
| Italy | 2 (6.5%) |
| Spain | 2 (6.5%) |
| Sweden | 2 (6.5%) |
| Belgium | 1 (3.2%) |
| China | 1 (3.2%) |
| France | 1 (3.2%) |
| Switzerland | 1 (3.2%) |
| Taiwan | 1 (3.2%) |
| a n (%) | |
| b Studies with multiple publications counted once (applies to 2 studies with 2 publications). | |
Comparators
Study Characteristics
Table 8. Selected characteristic of included studies.
| Study | N | Arm | ASA PSa | Dementia N (%)b |
Agec | MMSEc |
|---|---|---|---|---|---|---|
| HipFx — Randomized Clinical Trial | ||||||
| 64 | Std | NR | 80.0 (8.0) |
|||
| 62 | Exp | 78.0 (8.0) |
||||
| 69 | Std | NR | 0 (0) | 77.7 (7.1) |
||
| 68 | Exp | 0 (0) | 77.6 (8.3) |
|||
| 164 | Std | NR | 39 (23.8) | 82.6 (7.4) |
||
| 155 | Exp | 39 (25.2) | 81.1 (7.8) |
|||
| 82 | Std | NR | 0 (0) | 78.9 (7.3) |
||
| 80 | Exp | 0 (0) | 77.4 (8.2) |
|||
| 166 | Std | NR | 82 (49.4) | 85.0 {46-101} |
||
| 163 | Exp | 80 (49.1) | 84.0 {55-99} |
|||
| 199 | Std | NR | 26 (13.1) | 83.2 (6.4) |
||
| 198 | Exp | 27 (13.6) | 83.4 (5.4) |
|||
| 85 | Std | 1234 | 81.8 |
|||
| 70 | Exp | 79.5 |
||||
| GI/Abd — Randomized Clinical Trial | ||||||
| 63 | Std | NR | 6 (9.5) | 78.8 (7.8) |
||
| 53 | Exp | 3 (5.7) | 78.2 (7.4) |
|||
| Various — Randomized Clinical Trial | ||||||
| 149 | Std | NR | 77.6 (7.7) |
26.3 (3.9) | ||
| 148 | Exp | 77.5 (6.7) |
26.7 (3.0) | |||
| 133 | Std | NR | 77.4 (7.7) |
|||
| 127 | Exp | 77.4 (6.9) |
||||
| 105 | Std | NR | 5 (4.8) | 75.5 (6.3) |
||
| 104 | Exp | 2 (1.9) | 75.5 (6.6) |
|||
| HipFx — Nonrandomized Trial | ||||||
| 77 | Std | NR | 18 (23.4) | 81.1 (7.2) |
7.2 (3.9) | |
| 94 | Exp | 16 (17.0) | 80.4 (7.0) |
7.3 (3.9) | ||
| HipFx — Before-After/Time Series | ||||||
| 132 | Std | 1234 | 0 (0) | 82.0 (7.6) |
||
| 131 | Exp | 0 (0) | 81.1 (7.5) |
|||
| Std | NR | 82.9 |
||||
| 378 | Exp | 83.7 |
||||
| GI/Abd — Before-After/Time Series | ||||||
| 222 | Std | NR | 22 (9.9) | 77.0 {73-81} |
||
| 221 | Exp | 27 (12.2) | 77.0 {74-82} |
|||
| 63 | Std | 1234 | 81.4 {74-89} |
|||
| 86 | Exp | 80.6 {74-87} |
||||
| 117 | Std | NR | 82.0 {78-85} |
28 {26-29} | ||
| 90 | Exp | 79.0 {76-83} |
27 {25-29} | |||
| 54 | Std | NR | 75.0 {70-81} |
|||
| 18 | Exp | 80.0 {72-83} |
||||
| Cardiac — Before-After/Time Series | ||||||
| 183 | Std | NR | 29 (15.8) | 76.0 {73-78} |
||
| 183 | Exp | 32 (17.5) | 77.0 {73-80} |
|||
| Neuro — Before-After/Time Series | ||||||
| 25 | Std | NR | 73.0 (4.9) |
|||
| 100 | Exp | 73.6 (6.0) |
||||
| Urol — Before-After/Time Series | ||||||
| 101 | Std | NR | ||||
| 124 | Exp | |||||
| Other Ortho — Before-After/Time Series | ||||||
| 54 | Std | NR | 75.0 (6.1) |
|||
| 54 | Exp | 74.1 (6.2) |
||||
| 138 | Std | 123 | 66.0 (13.9) |
|||
| 128 | Exp | 68.0 (12.0) |
||||
| 59 | Std | 123 | 73.0 {68-77} |
|||
| 122 | Exp | 70.0 {64-77} |
||||
| Various — Before-After/Time Series | ||||||
| 30 | Std | 123 | 72.6 (8.3) |
|||
| 32 | Exp | 70.9 (6.3) |
||||
| 120 | Std | NR | 76.5 (4.9) |
26.6 (3.7) | ||
| 121 | Exp | 76.0 (5.1) |
27.3 (2.6) | |||
| 160 | Std | NR | 68.3 (11.2) |
|||
| 150 | Exp | 71.3 (10.6) |
||||
| 5275 | Std | 1234 | 60.3 (13.4) |
|||
| 3878 | Exp | 60.3 (13.7) |
||||
| 143 | Std | NR | 71.9 (6.4) |
|||
| 183 | Exp | 75.6 (6.8) |
||||
| 250 | Std | NR | 82.7 {78-87} |
|||
| 242 | Exp | 81.1 {78-86} |
||||
| GI/Abd — Retrospective Cohort | ||||||
| 107 | Std | 1234 | 4 (3.7) | 75.3 (5.1) |
||
| 203 | Exp | 27 (13.3) | 77.5 (4.8) |
|||
| Cardiac — Retrospective Cohort | ||||||
| 114 | Std | NR | 81.0 {77-83} |
|||
| 114 | Exp | 80.0 {79-82} |
||||
| Various — Prospective Cohort | ||||||
| 308 | Std | 234 | 80.0 (5.0) |
|||
| 158 | Exp | 80.0 (5.0) |
||||
| Exp: expanded preoperative evaluation; Std: standard preoperative evaluation; NR: not reported. | ||||||
| a ASA Physical Status. | ||||||
| b Blank if no information provided. | ||||||
| c Mean Med (SD)[Range]{IQR}. | ||||||
Evaluation/Intervention Components
Table 9. Components of expanded preoperative evaluation and interventions prompted.
| Assessments | Interventions | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Generala | Targeteda | Patient Education | Staff | |||||||||||||||||||
| HipFx — Randomized Clinical Trial | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
● |
◐ |
◐ |
◐ |
● | |||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◑ |
◐ |
◐ |
◐ |
||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
● |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
||||||||||
| GI/Abd — Randomized Clinical Trial | |||||||||||||||||||||||
| x | ◐ |
◐ |
◐ |
◐ |
● |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||
| Various — Randomized Clinical Trial | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
● |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||||||
◐ |
◐ |
● |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||||||
| HipFx — Nonrandomized Trial | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
● |
● |
● |
◐ |
◐ |
◐ |
◐ |
||||||||||||
| HipFx — Before-After/Time Series | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
● | ||||||||||||||||||
◐ |
◐ |
● | |||||||||||||||||||||
| GI/Abd — Before-After/Time Series | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||||||||||
| x | ◐ |
● |
● |
● |
● |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||||
| x | ● |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
● |
||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
||||||||||||||||||
| Cardiac — Before-After/Time Series | |||||||||||||||||||||||
● |
● |
● |
● |
● |
● |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||||
| Neuro — Before-After/Time Series | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||||||||||||
| Urol — Before-After/Time Series | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
||||||||||||||
| Other Ortho — Before-After/Time Series | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
||||||||||||||
◐ |
◐ |
◐ |
● |
◐ |
◐ |
◐ |
|||||||||||||||||
| x | ◐ |
● |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||||||
| Various — Before-After/Time Series | |||||||||||||||||||||||
◑ |
◐ |
◐ |
● |
● |
◐ |
||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
||||||||||||||||||
| x | ◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
|||||||||||||
| GI/Abd — Retrospective Cohort | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
● |
◐ |
◐ |
● | |||||||||
| Cardiac — Retrospective Cohort | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
||||||||||||||
| Various — Prospective Cohort | |||||||||||||||||||||||
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
◐ |
||||||||||||||||
| ERAS: enhanced recovery after surgery; CGA: comprehensive geriatric assessment; GI: gastrointestinal; Abd: abdominal; Suppl: supplement; ◐ expanded evaluation only; ● in expanded and standard evaluation arms; ◑ in standard evaluation arms only. | |||||||||||||||||||||||
| a When a comprehensive geriatric assessment (CGA) was conducted, all components were presumed included. | |||||||||||||||||||||||
| b Selective comprehensive geriatric assessment in standard arm. | |||||||||||||||||||||||
Intervention Frequency
| Patient interventions | |
| Intervention | N = 31a |
|---|---|
| Physical activity | 16 (52%) |
| Nutrition | 14 (45%) |
| Social Ties | 10 (32%) |
| Surgery/recovery expectations | 6 (19%) |
| Procedure | 2 (6.5%) |
| Palliative care discussion | 2 (6.5%) |
| a n (%) | |
| Staff interventions | |
| Intervention | N = 31a |
|---|---|
| Multidisciplinary | 26 (84%) |
| Treatment Plan | 24 (77%) |
| Geriatric Visits | 11 (35%) |
| Medication Review/Deprescribing | 13 (42%) |
| Nutritional Supplement | 9 (29%) |
| Rx/comorbidities | 8 (26%) |
| Active Delirium Screen | 4 (13%) |
| Haloperidol Prophylaxis | 3 (9.7%) |
| a n (%) | |
Delirium Incidence
Table 11. Delirium incidence with expanded versus standard preoperative evaluation.
| Study | N | Arm | Dementia N (%) |
Scale | Day(s)a | Surgery | Incidence Proportion | RR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| N (%) | 0 – 100% | ||||||||
| Randomized Clinical Trial | |||||||||
| 64 | Std | CAM | Stay | HipFx | 32 (50.0) | — | |||
| 62 | Exp | 20 (32.3) | 0.65 (0.42-1.00) | ||||||
| 164 | Std | 39 (23.8) | CAM | Stay | HipFx | 67 (40.9) | — | ||
| 155 | Exp | 39 (25.2) | 53 (34.2) | 0.84 (0.63-1.11) | |||||
| 166 | Std | 82 (49.4) | CAM | 10 | HipFx | 86 (51.8) | — | ||
| 163 | Exp | 80 (49.1) | 80 (49.1) | 0.95 (0.76-1.17) | |||||
| 85 | Std | DSM | Stay | HipFx | 27 (31.8) | — | |||
| 70 | Exp | 15 (21.4) | 0.67 (0.39-1.16) | ||||||
| 133 | Std | DOS/DSM | 10 | Various | 19 (14.3) | — | |||
| 127 | Exp | 12 (9.4) | 0.66 (0.33-1.31) | ||||||
| 91 | Std | 5 (4.8) | unspecified | Stay | Various | 22 (24.2) | — | ||
| 85 | Exp | 2 (1.9) | 9 (10.6) | 0.44 (0.21-0.90) | |||||
| Before-After/Time Series | |||||||||
| 132 | Std | 0 (0) | DSM/other | Stay | HipFx | 45 (34.1) | — | ||
| 131 | Exp | 0 (0) | 29 (22.1) | 0.65 (0.44-0.97) | |||||
| b | Stdc | DSM | Stay | HipFx | (29.0) | — | |||
| b | Stdd | (23.9) | — | ||||||
| b | Stde | (27.8) | — | ||||||
| 378 | Exp | 102 (27.0) | — | ||||||
| 54 | Std | unspecified | Stay | Other Ortho | 10 (18.5) | — | |||
| 54 | Exp | 3 (5.6) | 0.30 (0.09-1.03) | ||||||
| 222 | Std | 22 (9.9) | DOS/other | Stay | GI/Abd | 27 (12.2) | — | ||
| 221 | Exp | 27 (12.2) | 22 (10.0) | 0.82 (0.48-1.39) | |||||
| 120 | Std | CAM | 30 | Various | 16 (13.3) | — | |||
| 121 | Exp | 15 (12.4) | 0.93 (0.48-1.79) | ||||||
| 210 | Std | CAM | Stay | Various | 25 (11.9) | — | |||
| 182 | Exp | 12 (6.6) | 0.55 (0.29-1.07) | ||||||
| 25 | Std | unspecified | Stay | Neuro | 4 (16.0) | — | |||
| 100 | Exp | 18 (18.0) | 1.12 (0.42-3.03) | ||||||
| Retrospective Cohort | |||||||||
| 107 | Std | 4 (3.7) | CAM | Stay | GI/Abd | 31 (29.0) | — | ||
| 203 | Exp | 27 (13.3) | 23 (11.3) | 0.39 (0.24-0.64) | |||||
| 114 | Std | unspecified | Stay | Cardiac | 18 (15.8) | — | |||
| 114 | Exp | 13 (11.4) | 0.72 (0.37-1.40) | ||||||
| RR: risk ratio; CAM: Confusion Assessment Method; DI: Delirium Index; DRS: Delirium Rating Scale; DOS: delirium observation screening; DSM: Diagnostic and Statistical Manual of Mental Disorders; NR: not reported; HipFx: hip fracture; GI/Abd: gastrointestinal or abdominal. | |||||||||
| a Day(s) over which incidence proportion assessed. Stay indicates duration of hospitalization. | |||||||||
| b N = 611 for the 3 years of standard evaluation (2005-2007); outcomes reported by year separately. | |||||||||
| c 2005. | |||||||||
| d 2006. | |||||||||
| e 2007. | |||||||||
Pooled
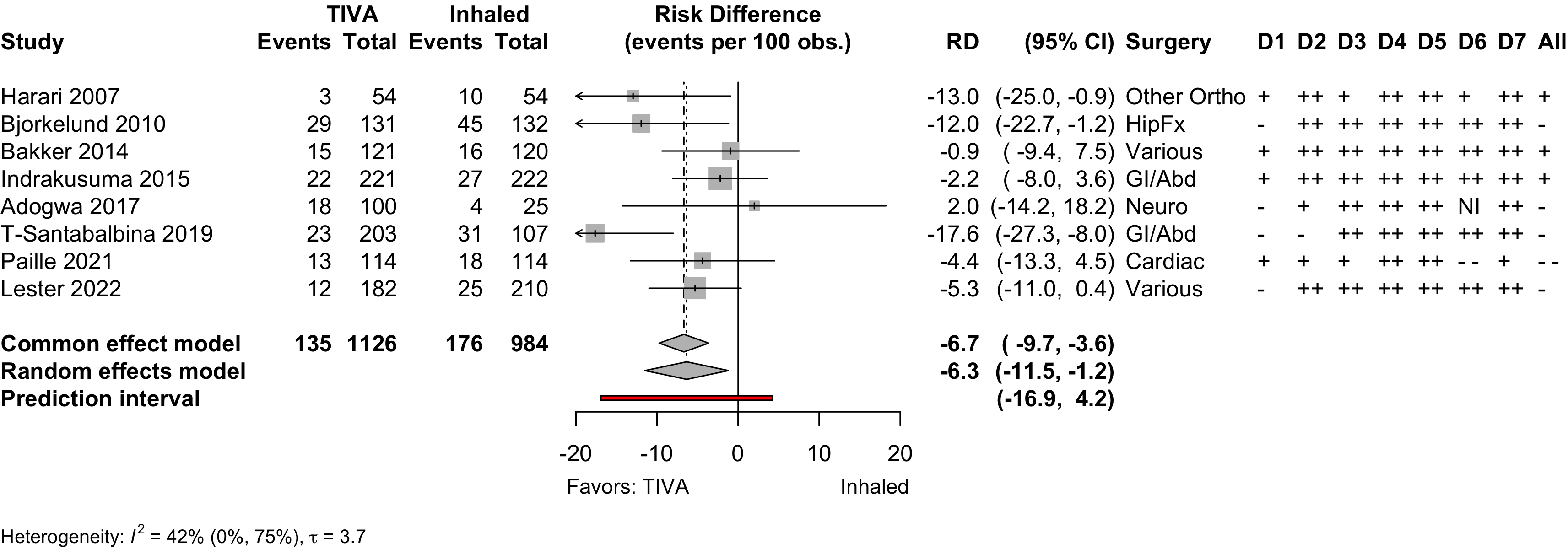
Randomized
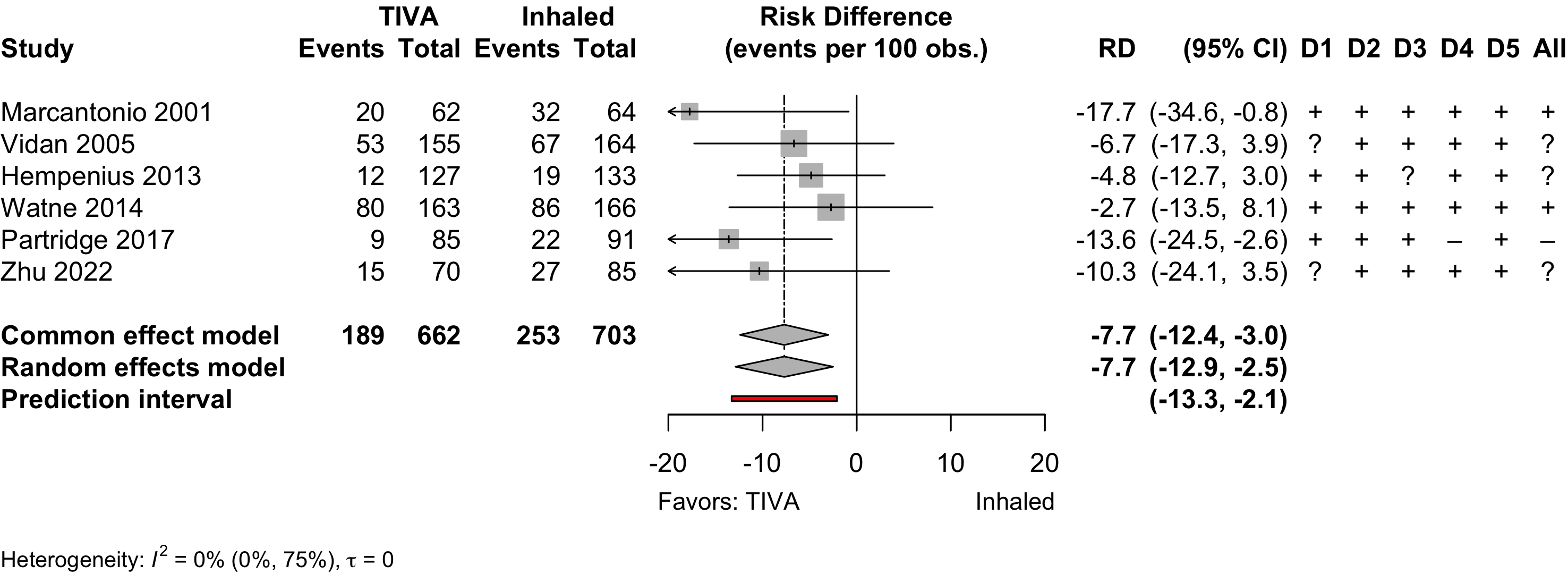
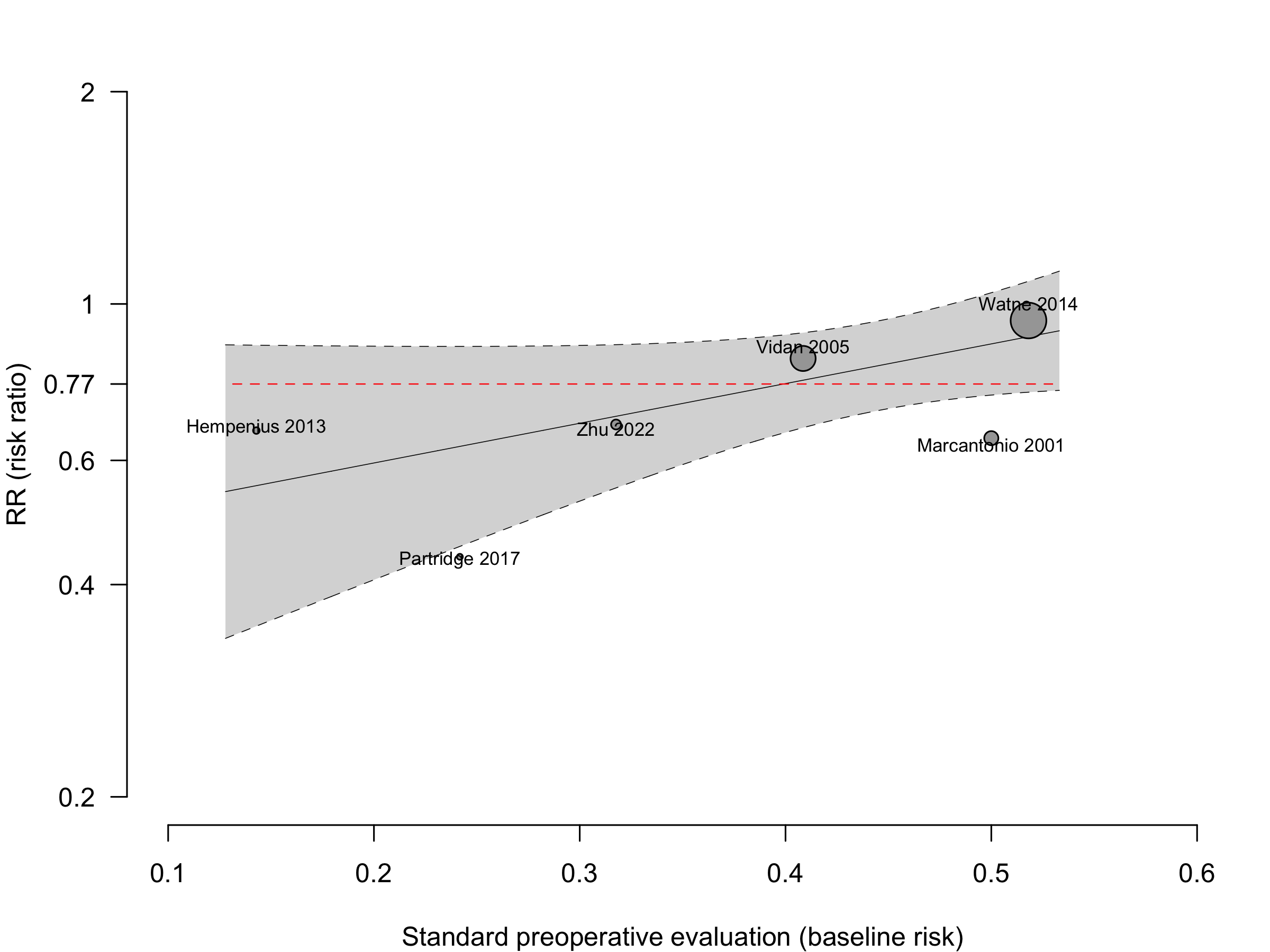
Figure 1. Delirium incidence following expanded compared with standard preoperative evaluation in randomized clinical trials.
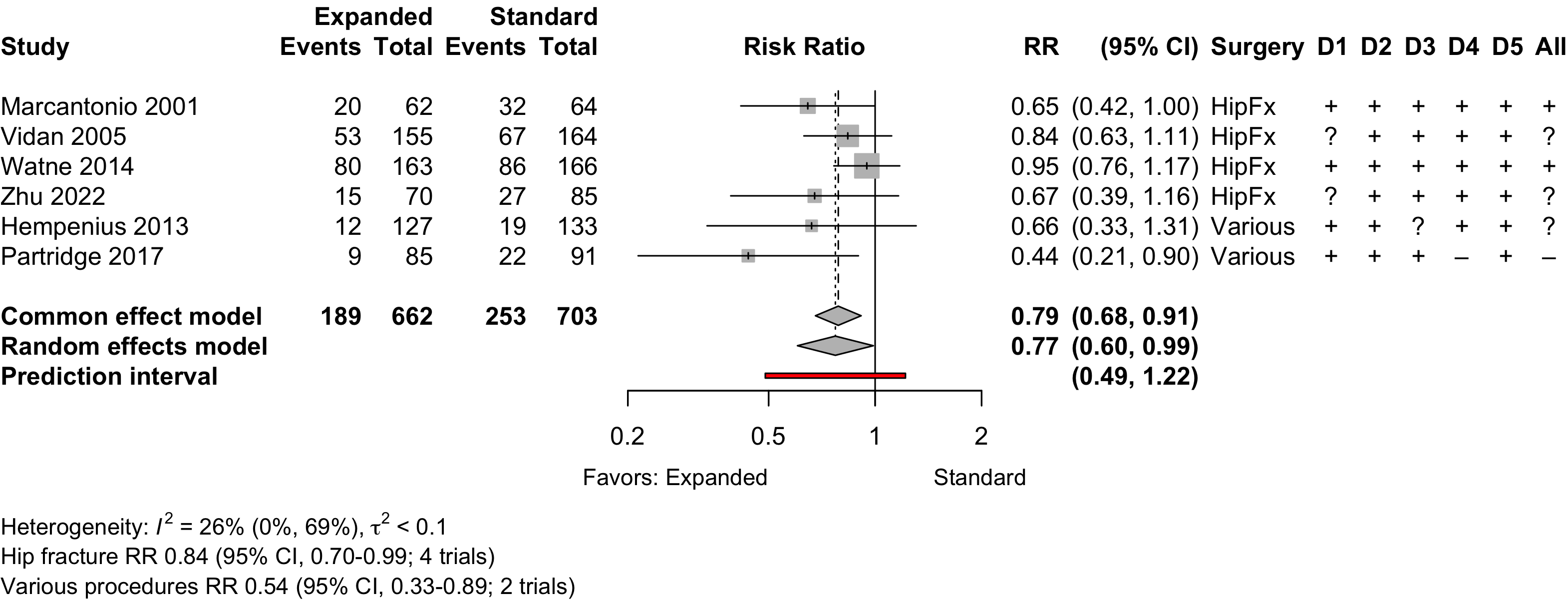
Risk of bias ratings: low +, some concerns ?, high – .
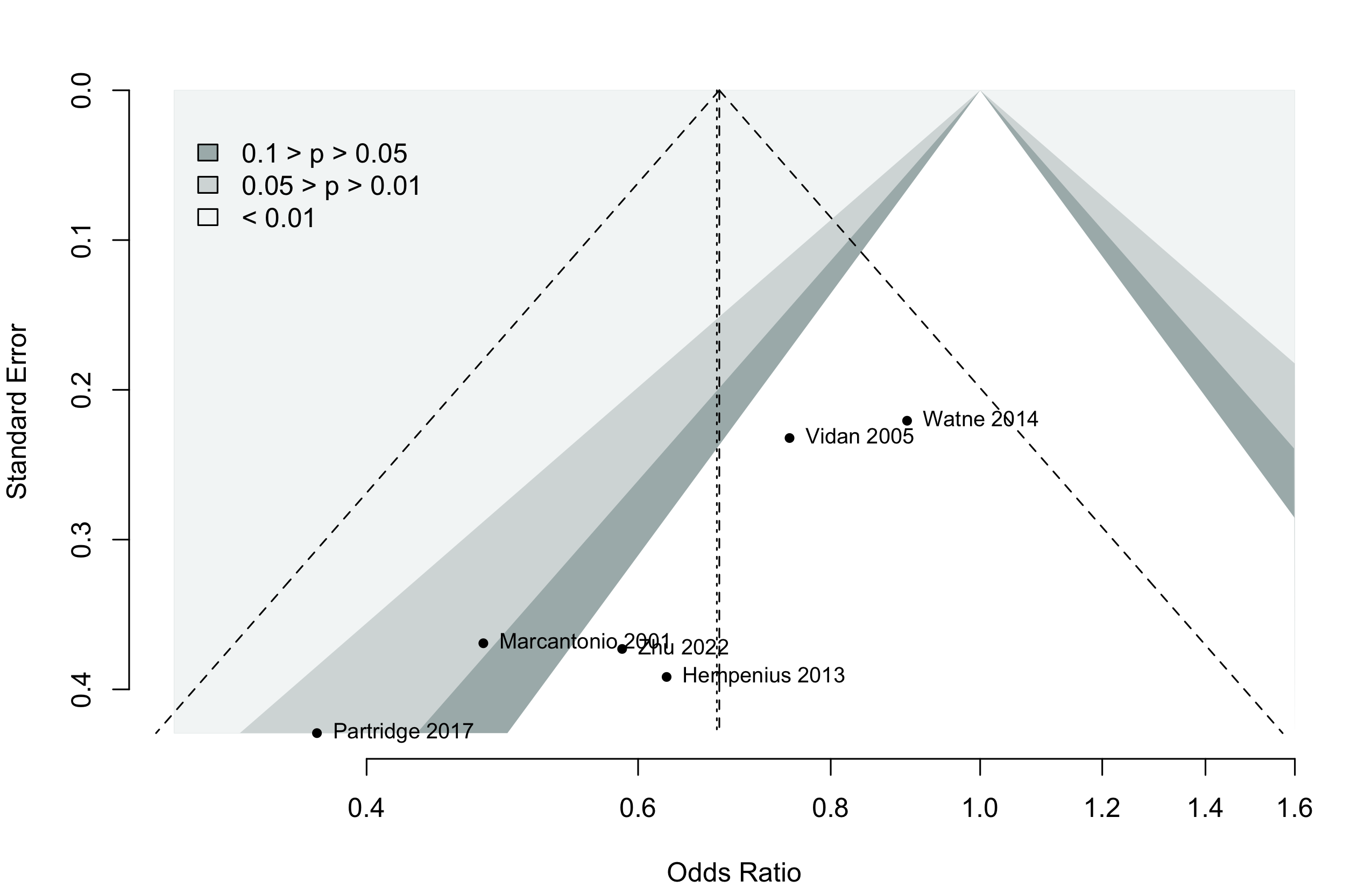
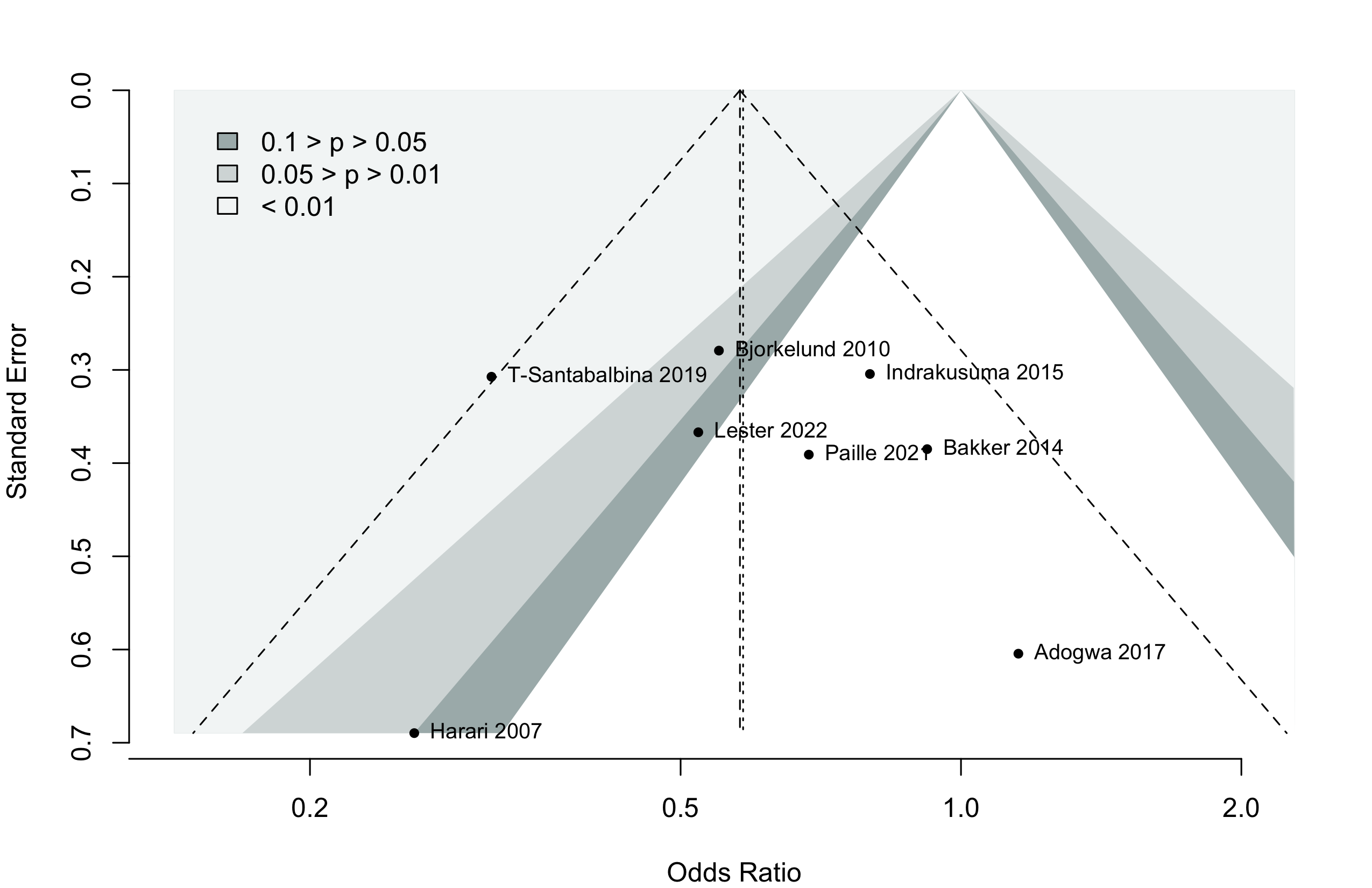
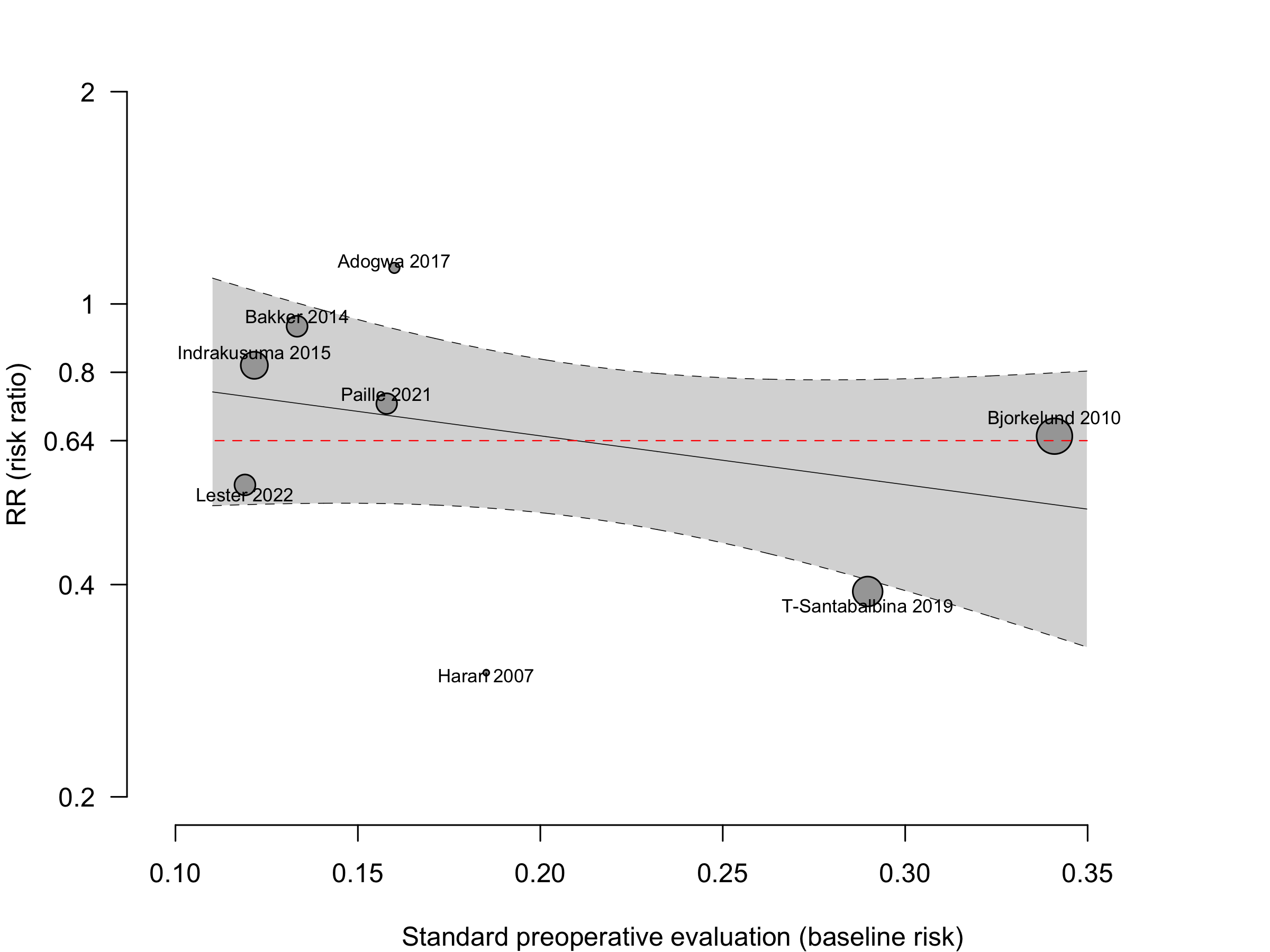
Too few studies to adequately examine small study effects, but funnel suggests potential exists.
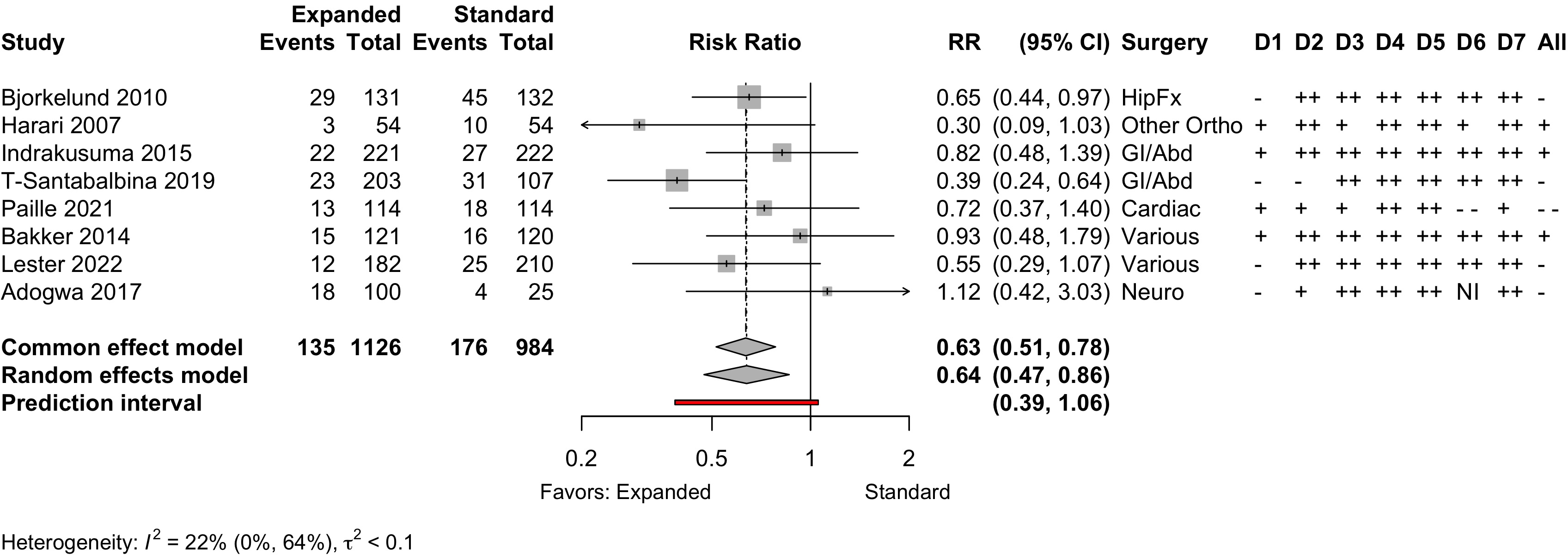
Nonrandomized
Figure 6. Delirium incidence following expanded compared with standard preoperative evaluation in nonrandomized studies.
Risk of bias ratings: low ++, moderate +, serious -, critical - - ; NI: no information.
Including Vochteloo 2011 assuming effectively equal numbers of participants studied each year, obtained RR 0.70 (95% CI, 0.53-0.95; PI, 0.35–1.42).
Pooling odds ratios using the adjusted result from Tarazona-Santabalbina 2019 yielded an OR 0.58 (95% CI, 0.39–0.88) versus using including the unadjusted data OR 0.58 (95%, 0.40–0.84).
Neurocognitive Disorder
<30 days
Table 12. Neurocognitive disorder <30 days incidence and ascertainment (expanded versus standard preoperative evaluation; randomized and nonrandomized designs).
| Study | N | Drug | Preop | Instrument | Neurocognitive Disorder <30 days | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MMSE | MMSE | DST | MoCA | Other | N (%) | 0 — 100% | RR (95% CI) | |||
| Before-After/Time Series — Various Procedures | ||||||||||
| 84 | Std | 26.6 (3.7) |
✓ |
16 (19.0) | — | |||||
| 96 | Exp | 27.3 (2.6) |
13 (13.5) | 0.71 (0.36-1.39) | ||||||
| Mini-Mental State Exam; DST: Digit Span Test; MoCA: Montreal Cognitive Assessment; RR: risk ratio. | ||||||||||
Neurocognitive Disorder
30 days to 1 year
Physical Function
Table 13. Physical functional status for expanded versus standard preoperative evaluation (randomized* and nonrandomized designs).
| Study | N | Arm | Agea | Scale | Range | Days | Ratinga | SMD (95% CI) | Surgery |
|---|---|---|---|---|---|---|---|---|---|
| <30 days | |||||||||
| 77 | Std | 81.1 (7.2) |
Katz Index | 0→18 | 8 | 12.9 (2.5) |
HipFx | ||
| 94 | Exp | 80.4 (7.0) |
12.0 (2.7) |
-0.34 (-0.65 to -0.04) | |||||
| 85 | Std | 81.8 |
Barthel Index | 0→100 | 21 | 51.7 |
HipFx | ||
| 70 | Exp | 79.5 |
50.5 |
-0.10 (-0.42 to 0.22) | |||||
| 80 | Std | 76.5 (4.9) |
GARS | 44→11 | 8 | b | Various | ||
| 98 | Exp | 76.0 (5.1) |
b | 0.29 (-0.01 to 0.59)c | |||||
| 133 | Std | 77.4 (7.7) |
SF-36 physical | 0→100 | 10 | 49.3 (7.0) |
Various | ||
| 127 | Exp | 77.4 (6.9) |
48.4 (9.1) |
-0.12 (-0.36 to 0.13) | |||||
| 30-90 days | |||||||||
| 169 | Std | 83.2 (6.4) |
Barthel Index | 0→20 | 30 | 14.2 (3.8) |
HipFx | ||
| 179 | Exp | 83.4 (5.4) |
14.5 (3.7) |
0.08 (-0.13 to 0.29) | |||||
| 69 | Std | 77.7 (7.1) |
SF-36 physical | 0→100 | 30 | 18.8 (21.2) |
HipFx | ||
| 68 | Exp | 77.6 (8.3) |
23.3 (20.4) |
0.22 (-0.12 to 0.55) | |||||
| 69 | Std | 77.7 (7.1) |
SF-36 physical | 0→100 | 90 | 28.2 (27.2) |
HipFx | ||
| 68 | Exp | 77.6 (8.3) |
48.4 (30.4) |
0.69 (0.35 to 1.04) | |||||
| 62 | Std | 76.5 (4.9) |
GARS | 44→11 | 90 | b | Various | ||
| 81 | Exp | 76.0 (5.1) |
b | -0.36 (-0.69 to -0.03)c | |||||
| 30 | Std | 72.6 (8.3) |
SF-36 physical | 0→100 | 42 | 37.6 (7.5) |
Various | ||
| 32 | Exp | 70.9 (6.3) |
39.0 (8.3) |
0.17 (-0.32 to 0.67) | |||||
| >90 days | |||||||||
| 183 | Std | 76.0 {73-78} |
SF-12 physical | 0→100 | 120 | 34 [0-43] |
Cardiac | ||
| 183 | Exp | 77.0 {73-80} |
36 [32-38] |
0.61 (0.40 to 0.82) | |||||
| 165 | Std | 83.2 (6.4) |
Barthel Index | 0→20 | 120 | 15.3 (3.7) |
HipFx | ||
| 168 | Exp | 83.4 (5.4) |
16.3 (3.7) |
0.27 (0.05 to 0.49) | |||||
| 120 | Std | 85.0 {46-101} |
Barthel Index | 0→20 | 120 | 16 [12-20] |
HipFx | ||
| 119 | Exp | 84.0 {55-99} |
17 [10-20] |
-0.09 (-0.34 to 0.16) | |||||
| 93 | Std | 85.0 {46-101} |
Barthel Index | 0→20 | 365 | 16 [11-19] |
HipFx | ||
| 97 | Exp | 84.0 {55-99} |
17 [10-19] |
-0.06 (-0.34 to 0.23) | |||||
| 30 | Std | 72.6 (8.3) |
SF-36 physical | 0→100 | 180 | 49.2 (10.4) |
Various | ||
| 32 | Exp | 70.9 (6.3) |
45.3 (10.9) |
-0.36 (-0.86 to 0.14) | |||||
| SMD: standardized mean difference; GARS: Groningen Activity Restriction Scale. | |||||||||
| * Randomized clinical trial. | |||||||||
| a Mean Med (SD)[Range]{IQR}. | |||||||||
| b Study reported only difference scores. | |||||||||
| c For scale reverse to be consistent with other studies. | |||||||||
Pooled
Randomized
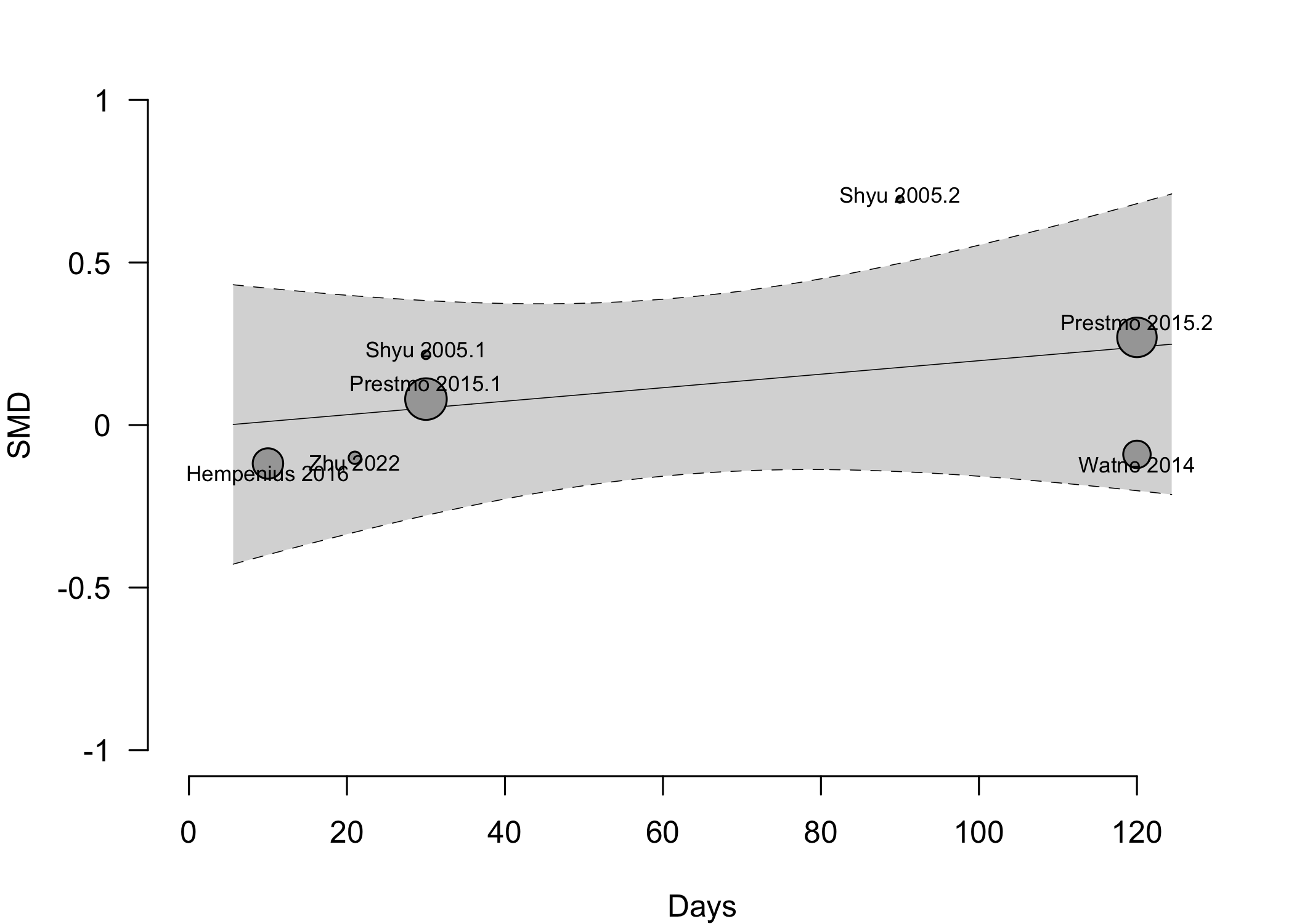
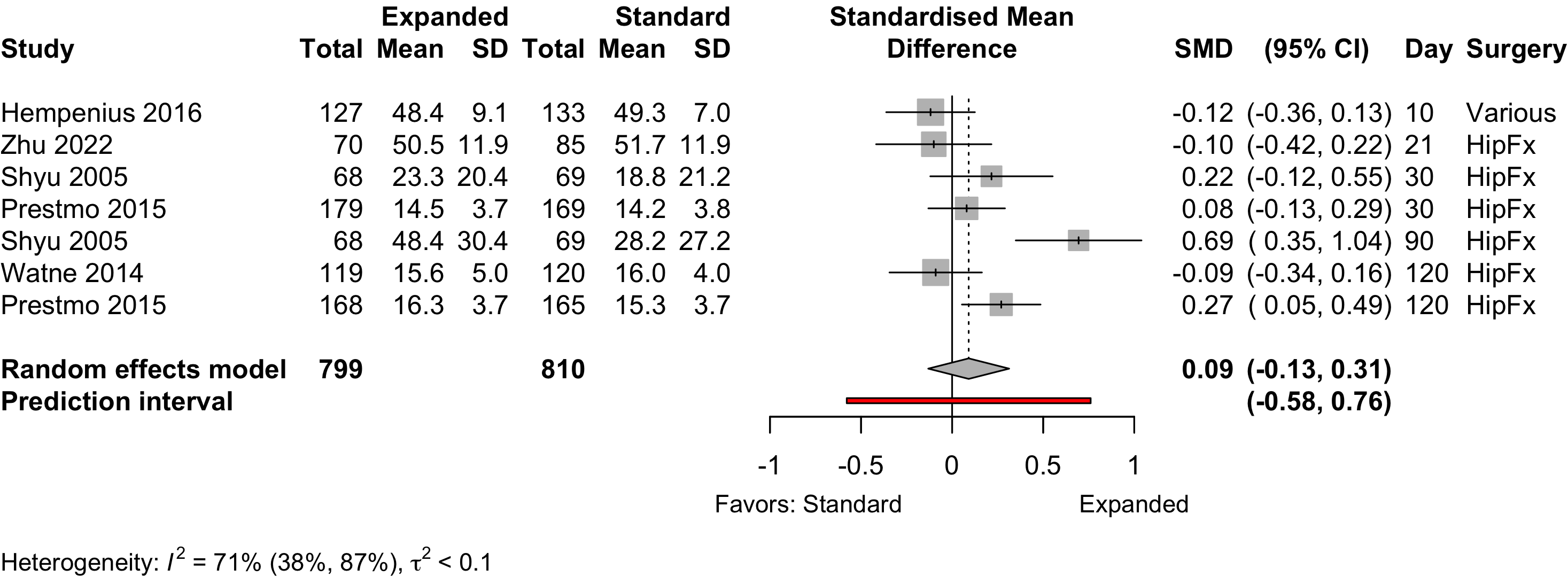
Figure 11. Pooled results for physical functional status for expanded versus standard preoperative evaluation from randomized clinical trials (higher is better).
Nonrandomized
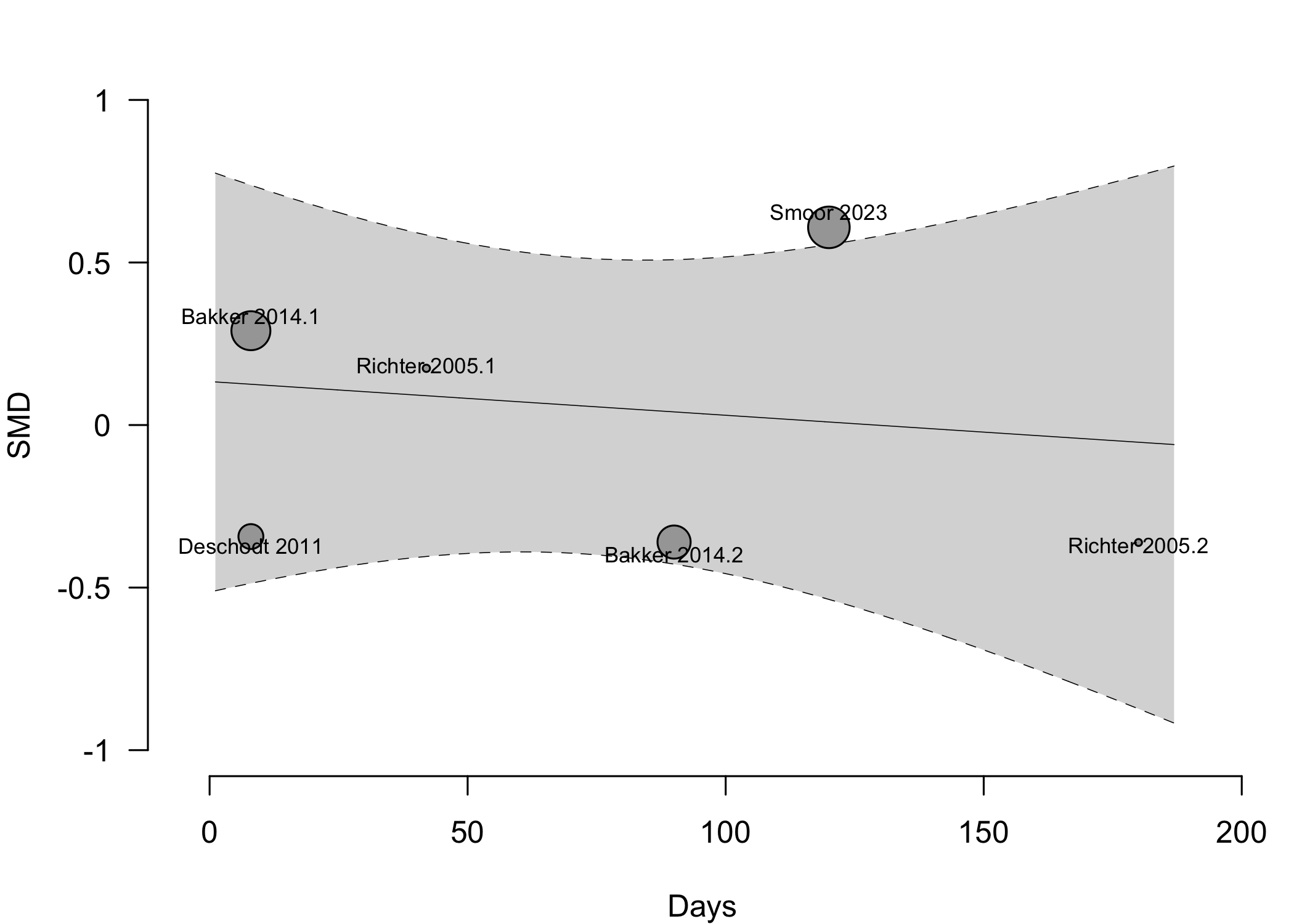
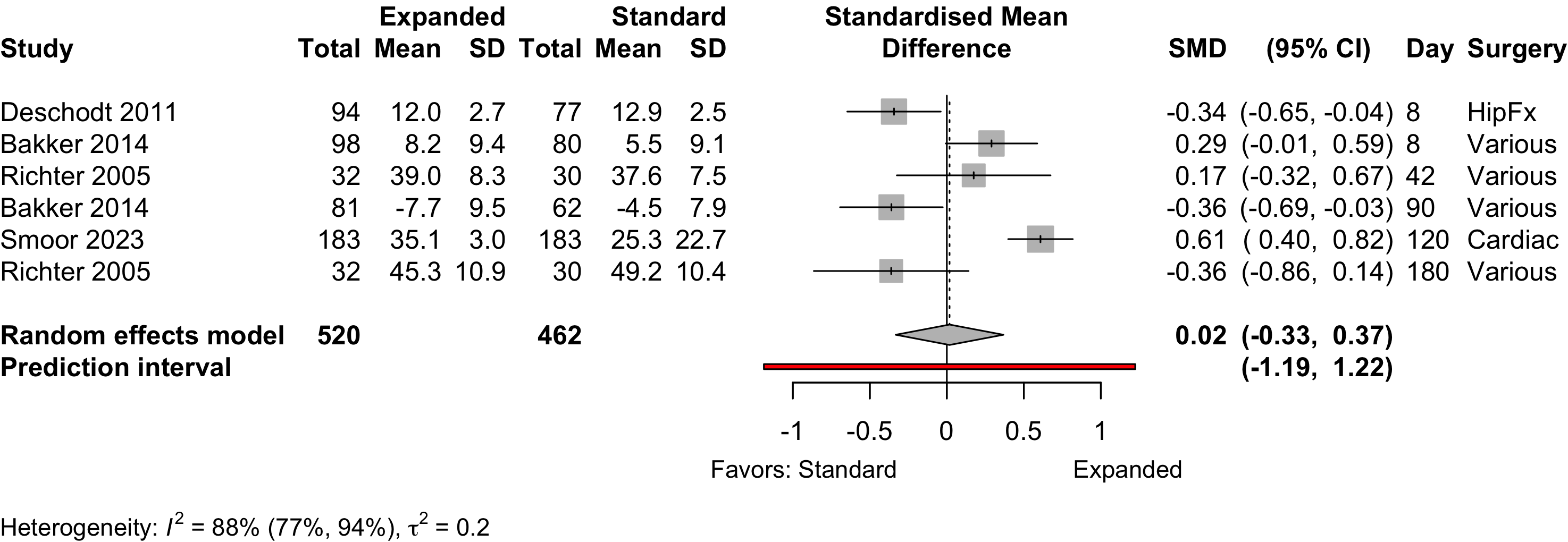
Figure 13. Pooled results for physical functional status for expanded versus standard preoperative evaluation from nonrandomized studies (higher is better).
Complications
Table 14. Complications reported in expanded versus standard preoperative evaluation — cardiac, pulmonary, and acute kidney injury (randomized and nonrandomized designs).
| Study | N | Arm | Agea | N (%) | 0 – 100% | RD (95% CI) | Surgery |
|---|---|---|---|---|---|---|---|
| MI – Randomized Clinical Trial | |||||||
| 164 | Std | 82.6 (7.4) |
1 (0.6) | — | HipFx | ||
| 155 | Exp | 81.1 (7.8) |
0 (0) | -0.6% (-2.3, 1.1) | |||
| MI – Before-After/Time Series | |||||||
| 132 | Std | 82.0 (7.6) |
5 (3.8) | — | HipFx | ||
| 131 | Exp | 81.1 (7.5) |
3 (2.3) | -1.5% (-5.6, 2.6) | |||
| 25 | Std | 73.0 (4.9) |
1 (4.0) | — | Neuro | ||
| 100 | Exp | 73.6 (6.0) |
1 (1.0) | -3.0% (-10.9, 4.9) | |||
| 250 | Std | 82.7 {78-87} |
0 (0) | — | Various | ||
| 242 | Exp | 81.1 {78-86} |
2 (0.8) | 0.8% (-0.6, 2.2) | |||
| 143 | Std | 71.9 (6.4) |
5 (3.5) | — | Various | ||
| 183 | Exp | 75.6 (6.8) |
3 (1.6) | -1.9% (-5.4, 1.7) | |||
| Cardiac Arrest – Randomized Clinical Trial | |||||||
| 91 | Std | 75.5 (6.3) |
5 (5.5) | — | Various | ||
| 85 | Exp | 75.5 (6.6) |
1 (1.2) | -4.3% (-9.5, 0.9) | |||
| Cardiac Arrest – Before-After/Time Series | |||||||
| 143 | Std | 71.9 (6.4) |
2 (1.4) | — | Various | ||
| 183 | Exp | 75.6 (6.8) |
1 (0.5) | -0.9% (-3.1, 1.3) | |||
| Other Cardiac – Randomized Clinical Trial | |||||||
| 166 | Std | 85.0 {46-101} |
19 (11.4) | — | HipFx | ||
| 163 | Exp | 84.0 {55-99} |
22 (13.5) | 2.1% (-5.1, 9.2) | |||
| 133 | Std | 77.6 (7.7) |
37 (27.8) | — | Various | ||
| 127 | Exp | 77.5 (6.7) |
40 (31.5) | 3.7% (-7.4, 14.8) | |||
| 91 | Std | 75.5 (6.3) |
25 (27.5) | — | Various | ||
| 85 | Exp | 75.5 (6.6) |
7 (8.2) | -19.2% (-30.1, -8.4) | |||
| Other Cardiac – Before-After/Time Series | |||||||
| 117 | Std | 82.0 {78-85} |
25 (21.4) | — | GI/Abd | ||
| 90 | Exp | 79.0 {76-83} |
16 (17.8) | -3.6% (-14.4, 7.3) | |||
| 222 | Std | 77.0 {73-81} |
28 (12.6) | — | GI/Abd | ||
| 221 | Exp | 77.0 {74-82} |
32 (14.5) | 1.9% (-4.5, 8.2) | |||
| 63 | Std | 81.4 {74-89} |
5 (7.9) | — | GI/Abd | ||
| 86 | Exp | 80.6 {74-87} |
0 (0) | -7.9% (-15.0, -0.9) | |||
| 132 | Std | 82.0 (7.6) |
11 (8.3) | — | HipFx | ||
| 131 | Exp | 81.1 (7.5) |
7 (5.3) | -3.0% (-9.1, 3.1) | |||
| 143 | Std | 71.9 (6.4) |
45 (31.5) | — | Various | ||
| 183 | Exp | 75.6 (6.8) |
40 (21.9) | -9.6% (-19.3, 0.1) | |||
| Acute Kidney Injury – Randomized Clinical Trial | |||||||
| 166 | Std | 85.0 {46-101} |
2 (1.2) | — | HipFx | ||
| 163 | Exp | 84.0 {55-99} |
6 (3.7) | 2.5% (-0.9, 5.8) | |||
| 133 | Std | 77.6 (7.7) |
2 (1.5) | — | Various | ||
| 127 | Exp | 77.5 (6.7) |
5 (3.9) | 2.4% (-1.5, 6.4) | |||
| Acute Kidney Injury – Before-After/Time Series | |||||||
| 117 | Std | 82.0 {78-85} |
41 (35.0) | — | GI/Abd | ||
| 90 | Exp | 79.0 {76-83} |
25 (27.8) | -7.3% (-19.9, 5.4) | |||
| 132 | Std | 82.0 (7.6) |
2 (1.5) | — | HipFx | ||
| 131 | Exp | 81.1 (7.5) |
1 (0.8) | -0.8% (-3.3, 1.8) | |||
| 143 | Std | 71.9 (6.4) |
13 (9.1) | — | Various | ||
| 183 | Exp | 75.6 (6.8) |
15 (8.2) | -0.9% (-7.1, 5.3) | |||
| Pulmonary – Randomized Clinical Trial | |||||||
| 166 | Std | 85.0 {46-101} |
13 (7.8) | — | HipFx | ||
| 163 | Exp | 84.0 {55-99} |
21 (12.9) | 5.1% (-1.5, 11.6) | |||
| 133 | Std | 77.6 (7.7) |
27 (20.3) | — | Various | ||
| 127 | Exp | 77.5 (6.7) |
31 (24.4) | 4.1% (-6.0, 14.2) | |||
| 91 | Std | 75.5 (6.3) |
13 (14.3) | — | Various | ||
| 85 | Exp | 75.5 (6.6) |
8 (9.4) | -4.9% (-14.4, 4.6) | |||
| Pulmonary – Retrospective Cohort | |||||||
| 114 | Std | 81.0 {77-83} |
37 (32.5) | — | Cardiac | ||
| 114 | Exp | 80.0 {79-82} |
32 (28.1) | -4.4% (-16.3, 7.5) | |||
| Pneumonia – Randomized Clinical Trial | |||||||
| 164 | Std | 82.6 (7.4) |
6 (3.7) | — | HipFx | ||
| 155 | Exp | 81.1 (7.8) |
6 (3.9) | 0.2% (-4.0, 4.4) | |||
| 91 | Std | 75.5 (6.3) |
12 (13.2) | — | Various | ||
| 85 | Exp | 75.5 (6.6) |
8 (9.4) | -3.8% (-13.1, 5.5) | |||
| Pneumonia – Before-After/Time Series | |||||||
| 117 | Std | 82.0 {78-85} |
26 (22.2) | — | GI/Abd | ||
| 90 | Exp | 79.0 {76-83} |
10 (11.1) | -11.1% (-21.1, -1.2) | |||
| 222 | Std | 77.0 {73-81} |
31 (14.0) | — | GI/Abd | ||
| 221 | Exp | 77.0 {74-82} |
37 (16.7) | 2.8% (-3.9, 9.5) | |||
| 132 | Std | 82.0 (7.6) |
3 (2.3) | — | HipFx | ||
| 131 | Exp | 81.1 (7.5) |
6 (4.6) | 2.3% (-2.1, 6.7) | |||
| 25 | Std | 73.0 (4.9) |
1 (4.0) | — | Neuro | ||
| 100 | Exp | 73.6 (6.0) |
5 (5.0) | 1.0% (-7.8, 9.8) | |||
| 54 | Std | 75.0 (6.1) |
11 (20.4) | — | Other Ortho | ||
| 54 | Exp | 74.1 (6.2) |
2 (3.7) | -16.7% (-28.5, -4.8) | |||
| 250 | Std | 82.7 {78-87} |
2 (0.8) | — | Various | ||
| 242 | Exp | 81.1 {78-86} |
1 (0.4) | -0.4% (-1.8, 1.0) | |||
| 143 | Std | 71.9 (6.4) |
2 (1.4) | — | Various | ||
| 183 | Exp | 75.6 (6.8) |
3 (1.6) | 0.2% (-2.4, 2.9) | |||
| Respiratory Failure – Before-After/Time Series | |||||||
| 143 | Std | 71.9 (6.4) |
21 (14.7) | — | Various | ||
| 183 | Exp | 75.6 (6.8) |
16 (8.7) | -5.9% (-13.0, 1.2) | |||
| Ventilator >48hr – Before-After/Time Series | |||||||
| 143 | Std | 71.9 (6.4) |
2 (1.4) | — | Various | ||
| 183 | Exp | 75.6 (6.8) |
0 (0) | -1.4% (-3.7, 0.9) | |||
| PE – Before-After/Time Series | |||||||
| 132 | Std | 82.0 (7.6) |
2 (1.5) | — | HipFx | ||
| 131 | Exp | 81.1 (7.5) |
2 (1.5) | 0.0% (-2.9, 3.0) | |||
| 25 | Std | 73.0 (4.9) |
0 (0) | — | Neuro | ||
| 100 | Exp | 73.6 (6.0) |
2 (2.0) | 2.0% (-4.1, 8.1) | |||
| 54 | Std | 75.0 (6.1) |
2 (3.7) | — | Other Ortho | ||
| 54 | Exp | 74.1 (6.2) |
0 (0) | -3.7% (-9.8, 2.3) | |||
| 143 | Std | 71.9 (6.4) |
1 (0.7) | — | Various | ||
| 183 | Exp | 75.6 (6.8) |
2 (1.1) | 0.4% (-1.6, 2.4) | |||
| Acute Kidney Injury – Retrospective Cohort | |||||||
| 114 | Std | 81.0 {77-83} |
44 (38.6) | — | Cardiac | ||
| 114 | Exp | 80.0 {79-82} |
33 (28.9) | -9.6% (-21.9, 2.6) | |||
| RD: risk difference; MI: myocardial infarction; GI: gastrointestinal; Abd: abdominal; Ortho: orthopedic; Neuro: neurologic. | |||||||
| a Mean Med (SD)[Range]{IQR}. | |||||||
| b Unspecified. | |||||||
| c Arrhythmia, cardiac failure. | |||||||
| d Acute coronary syndrome, heart failure, tachyarrhythmia, or bradyarrhythmia. | |||||||
| e Cardiac failure. | |||||||
| f MI, cardiac arrest, ventricular fibrillation, dysrhythmia, or heart failure. | |||||||
| g Pneumonia, infectious exacerbation of chronic obstructive pulmonary disease (COPD). | |||||||
| h Pulmonary embolism or pneumothorax. | |||||||
Pooled
Myocardial Infarction
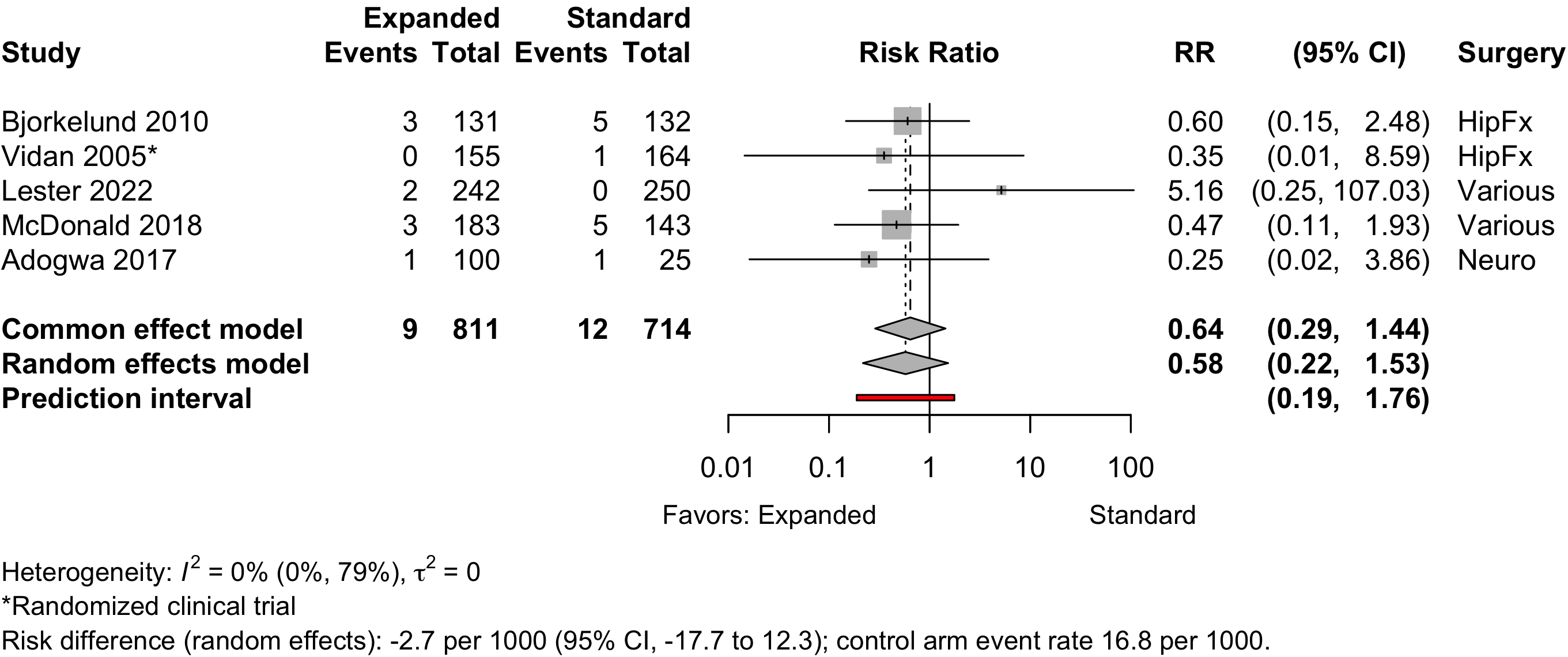
Figure 15. Risk ratio for myocardial infarction (randomized and nonrandomized designs).
Cardiac Arrest
Figure 16. Risk ratio for cardiac arrest (randomized and nonrandomized designs).
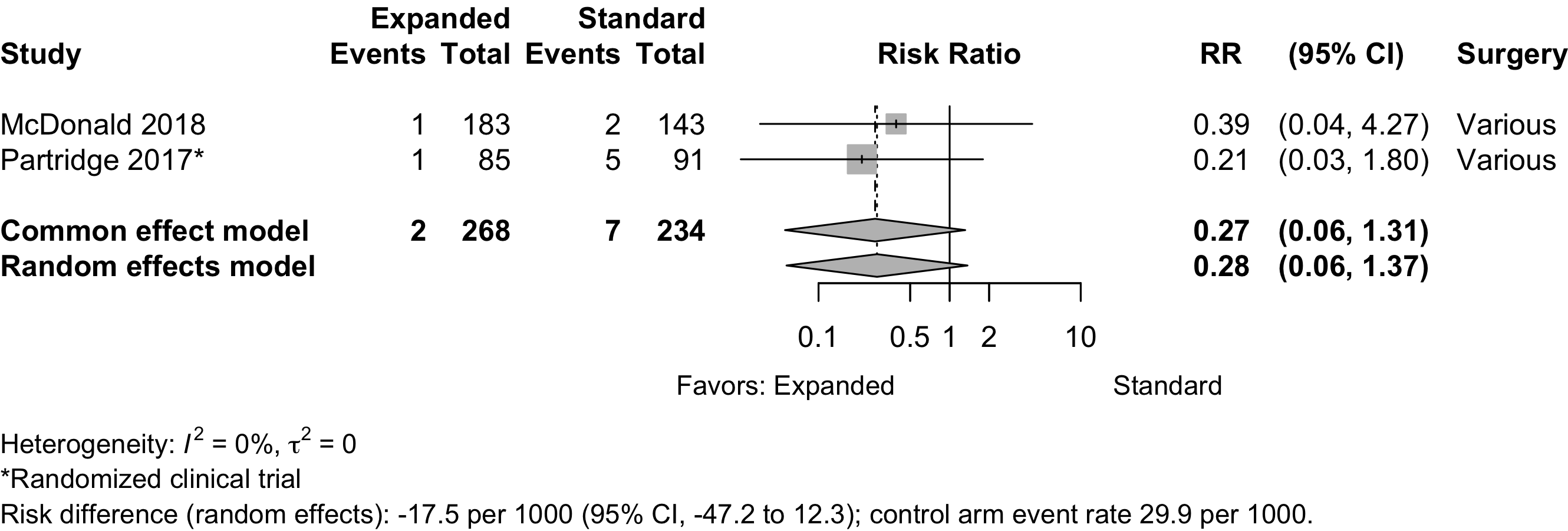
Acute Kidney Injury
Figure 17. Risk ratio for renal complications (expanded versus standard preoperative evaluation; randomized clinical trials).
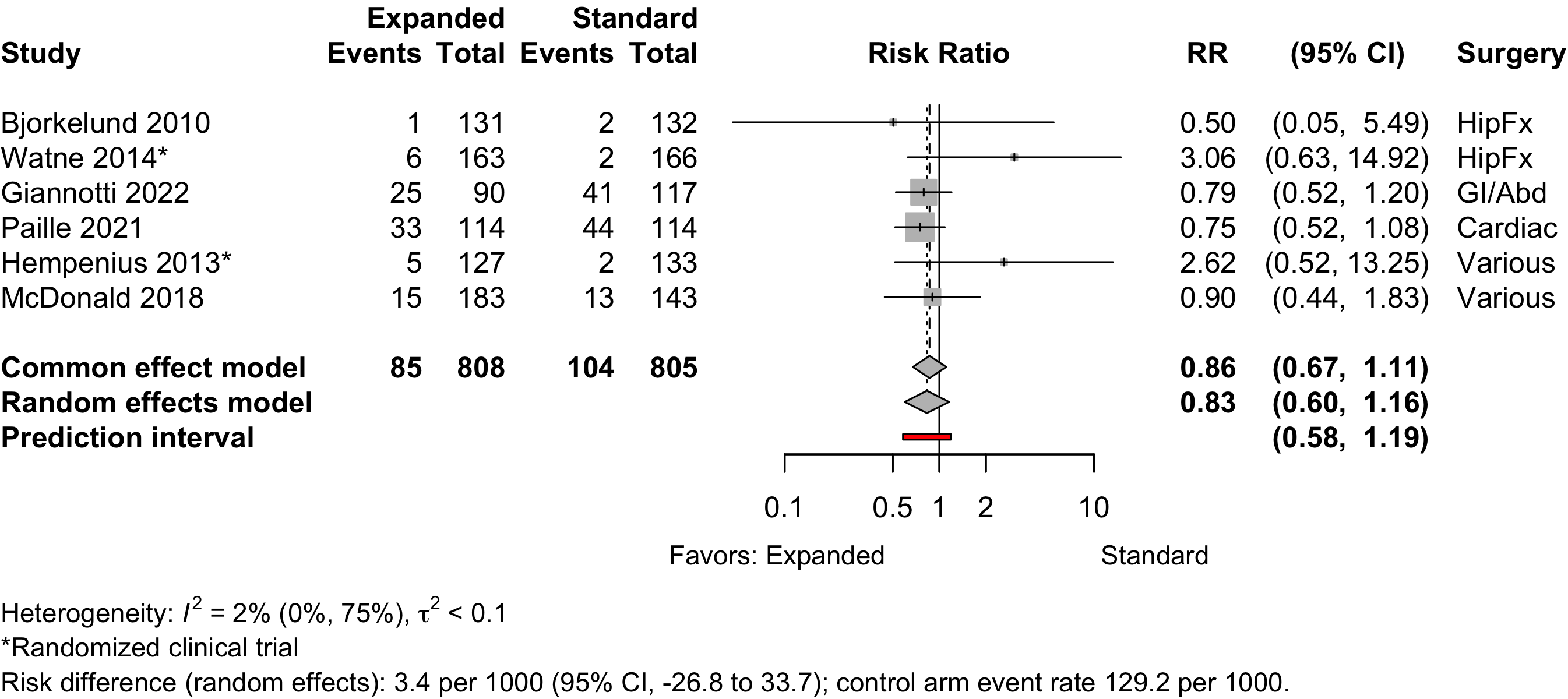
Pneumonia
Figure 18. Risk ratio for pneumonia (randomized and nonrandomized designs).
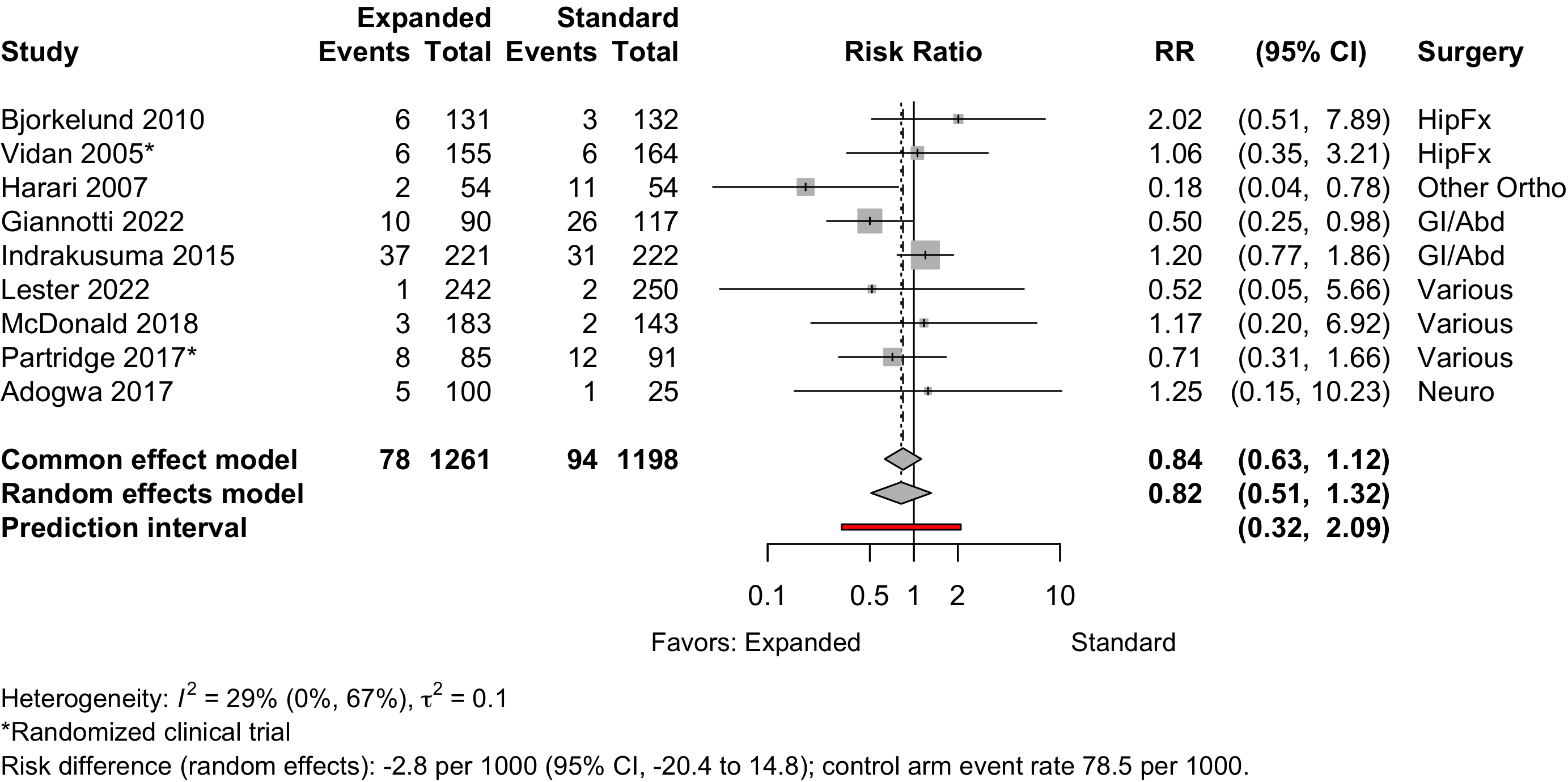
Pulmonary Embolism
Figure 19. Risk ratio for pneumonia (randomized and nonrandomized designs).
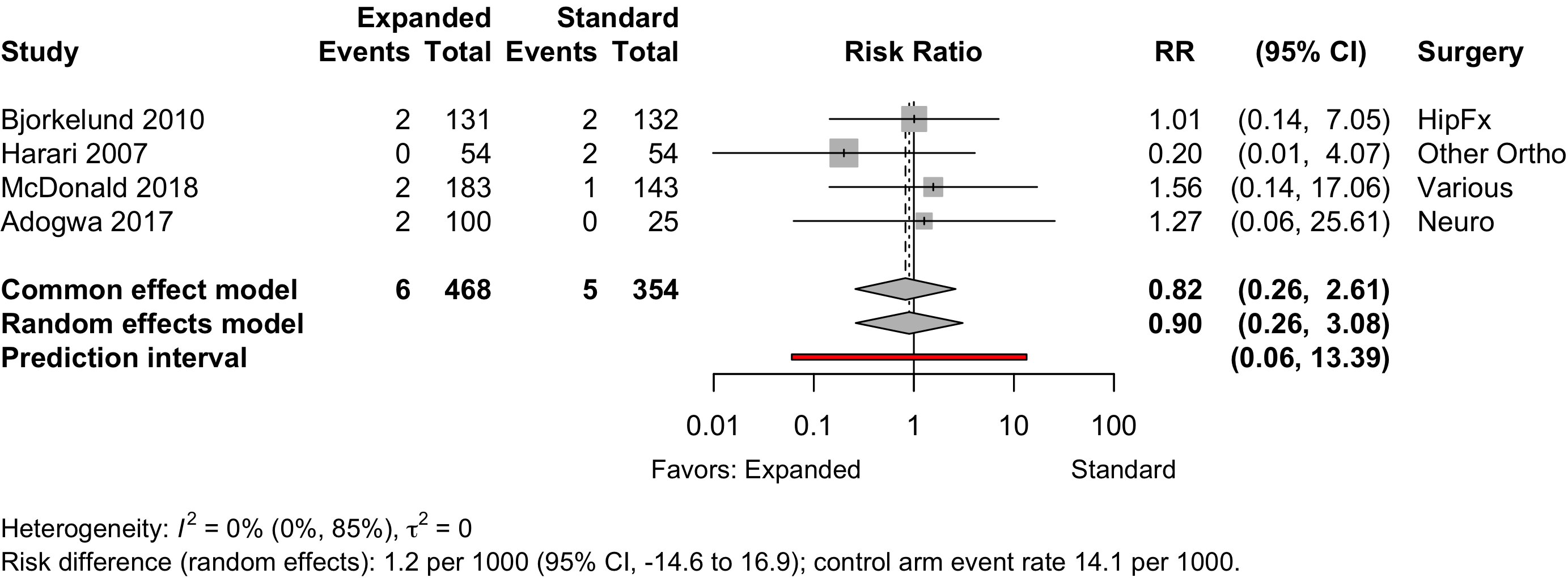
Patient Satisfaction
Table 15. Patient satisfaction according to expanded or standard preoperative evaluation.
| Study | N | Anesth | ASA | Agea | N (%) | 0 – 100% | RD (95% CI) |
|---|---|---|---|---|---|---|---|
| PS | |||||||
| Various (pelvic floor) | |||||||
| 30 | Std | 123 | 72.6 (8.3) |
29 (96.7)b | — | ||
| 32 | Exp | 70.9 (6.3) |
32 (100)b | 3.3% (-5.3, 12.0) | |||
| Std: standard evaluation; Exp: expanded evaluation; ASA PS: ASA Physical Status; RD: risk difference. | |||||||
| a Mean (SD). | |||||||
| b High satisfaction. | |||||||
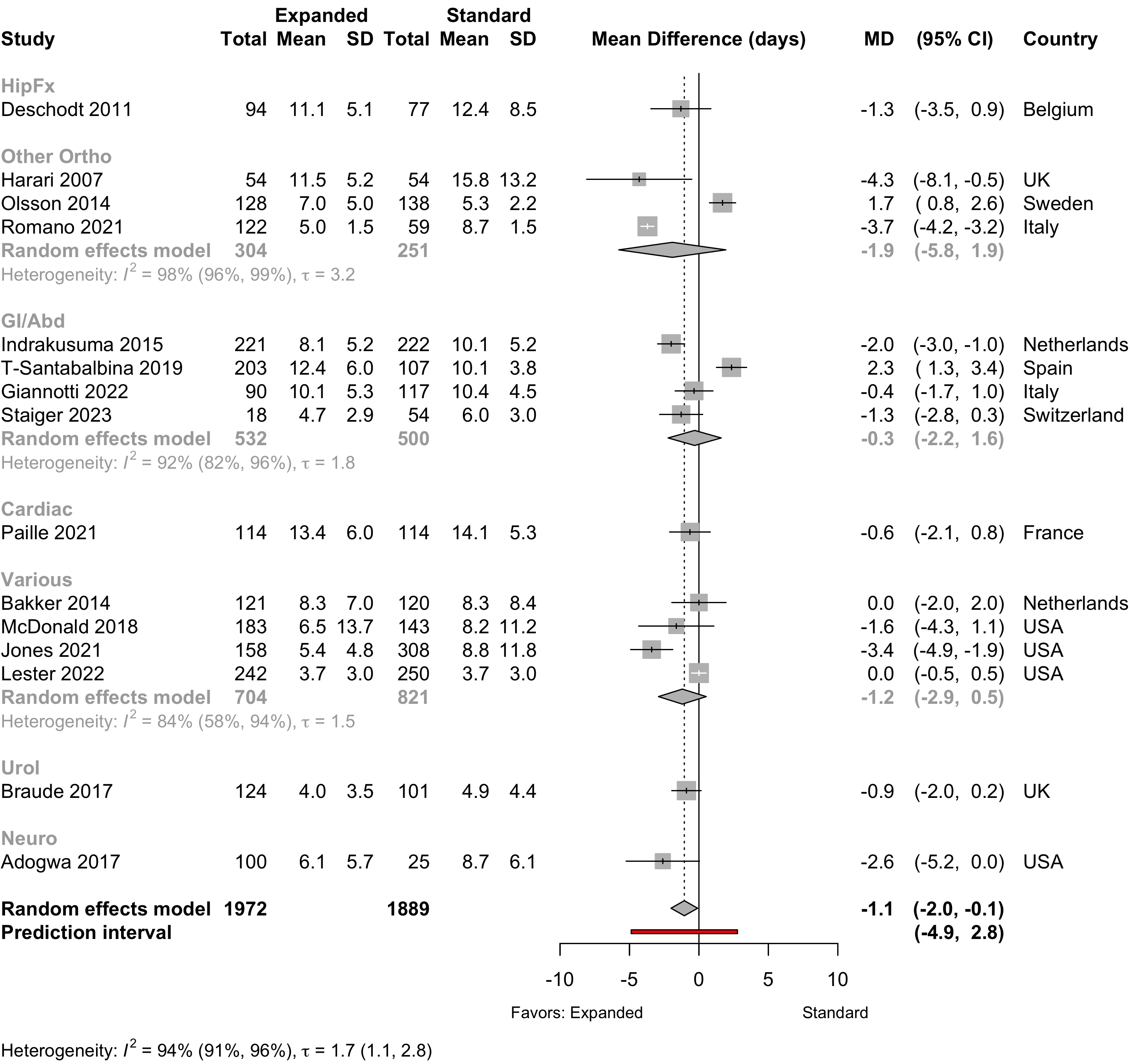
Length of Stay
Table 16. Length of stay according to procedure classification and type of preoperative evaluation.
| Study | N | Arm | PSa | Ageb | LOSb | 0 – 25 days | Country |
|---|---|---|---|---|---|---|---|
| Randomized Clinical Trial - HipFx | |||||||
| 64 | Std | NR | 80.0 (8.0) |
5.0 {3-7} |
USA | ||
| 62 | Exp | NR | 78.0 (8.0) |
5.0 {3-7} |
|||
| 69 | Std | NR | 77.7 (7.1) |
10.2 (6.5) |
Taiwan | ||
| 68 | Exp | NR | 77.6 (8.3) |
10.1 (3.7) |
|||
| 164 | Std | NR | 82.6 (7.4) |
18.0 {13-24} |
Spain | ||
| 155 | Exp | NR | 81.1 (7.8) |
16.0 {13-19} |
|||
| 166 | Std | NR | 85.0 {46-101} |
8.0 {5-11} |
Norway | ||
| 163 | Exp | NR | 84.0 {55-99} |
11.0 {8-15} |
|||
| 199 | Std | NR | 83.2 (6.4) |
11.0 (0.5) |
Norway | ||
| 198 | Exp | NR | 83.4 (5.4) |
12.6 (0.4) |
|||
| 85 | Std | 1234 | 81.8 |
23.6 |
China | ||
| 70 | Exp | 1234 | 79.5 |
20.8 |
|||
| Nonrandomized Trial - HipFx | |||||||
| 77 | Std | NR | 81.1 (7.2) |
12.4 (8.5) |
Belgium | ||
| 94 | Exp | NR | 80.4 (7.0) |
11.1 (5.1) |
|||
| Before-After/Time Series - Other Ortho | |||||||
| 54 | Std | NR | 75.0 (6.1) |
15.8 (13.2) |
UK | ||
| 54 | Exp | NR | 74.1 (6.2) |
11.5 (5.2) |
|||
| 138 | Std | 123 | 66.0 (13.9) |
5.3 (2.2) |
Sweden | ||
| 128 | Exp | 123 | 68.0 (12.0) |
7.0 (5.0) |
|||
| 59 | Std | 123 | 73.0 {68-77} |
8.0 {8-10} |
Italy | ||
| 122 | Exp | 123 | 70.0 {64-77} |
5.0 {4-6} |
|||
| Randomized Clinical Trial - GI/Abd | |||||||
| 63 | Std | NR | 78.8 (7.8) |
8.0 |
Norway | ||
| 53 | Exp | NR | 78.2 (7.4) |
8.0 |
|||
| Before-After/Time Series - GI/Abd | |||||||
| 222 | Std | NR | 77.0 {73-81} |
9.0 {7-14} |
Netherlands | ||
| 221 | Exp | NR | 77.0 {74-82} |
7.0 {5-12} |
|||
| 117 | Std | NR | 82.0 {78-85} |
9.0 {8-14} |
Italy | ||
| 90 | Exp | NR | 79.0 {76-83} |
9.0 {7-14} |
|||
| 54 | Std | NR | 75.0 {70-81} |
6.0 {4-8} |
Switzerland | ||
| 18 | Exp | NR | 80.0 {72-83} |
4.0 {3-7} |
|||
| Retrospective Cohort - GI/Abd | |||||||
| 107 | Std | 1234 | 75.3 (5.1) |
9.0 {8-13} |
Spain | ||
| 203 | Exp | 1234 | 77.5 (4.8) |
11.0 {9-17} |
|||
| Retrospective Cohort - Cardiac | |||||||
| 114 | Std | NR | 81.0 {77-83} |
13.0 {11-18} |
France | ||
| 114 | Exp | NR | 80.0 {79-82} |
12.0 {10-18} |
|||
| Before-After/Time Series - Neuro | |||||||
| 25 | Std | NR | 73.0 (4.9) |
8.7 (6.1) |
USA | ||
| 100 | Exp | NR | 73.6 (6.0) |
6.1 (5.7) |
|||
| Before-After/Time Series - Urol | |||||||
| 101 | Std | NR | 4.9 (4.4) |
UK | |||
| 124 | Exp | NR | 4.0 (3.5) |
||||
| Randomized Clinical Trial - Various | |||||||
| 149 | Std | NR | 77.6 (7.7) |
8.0 [1-44] |
Netherlands | ||
| 148 | Exp | NR | 77.5 (6.7) |
8.0 [1-135] |
|||
| 105 | Std | NR | 75.5 (6.3) |
5.5c |
UK | ||
| 104 | Exp | NR | 75.5 (6.6) |
3.3c |
|||
| Before-After/Time Series - Various | |||||||
| 120 | Std | NR | 76.5 (4.9) |
8.3 (8.4) |
Netherlands | ||
| 121 | Exp | NR | 76.0 (5.1) |
8.3 (7.0) |
|||
| 143 | Std | NR | 71.9 (6.4) |
6.0 [1-60] |
USA | ||
| 183 | Exp | NR | 75.6 (6.8) |
4.0 [1-75] |
|||
| 250 | Std | NR | 82.7 {78-87} |
3.0 {2-6} |
USA | ||
| 242 | Exp | NR | 81.1 {78-86} |
3.0 {2-6} |
|||
| Prospective Cohort - Various | |||||||
| 308 | Std | 234 | 80.0 (5.0) |
8.8 (11.8) |
USA | ||
| 158 | Exp | 234 | 80.0 (5.0) |
5.4 (4.8) |
|||
| NR: not reported | |||||||
| a ASA Physical Status. | |||||||
| b Mean Med (SD)[Range]{IQR}. | |||||||
| c Geometric mean. | |||||||
Pooled
Randomized
Figure 20. Mean difference in lengths of stay in randomized clinical trials.
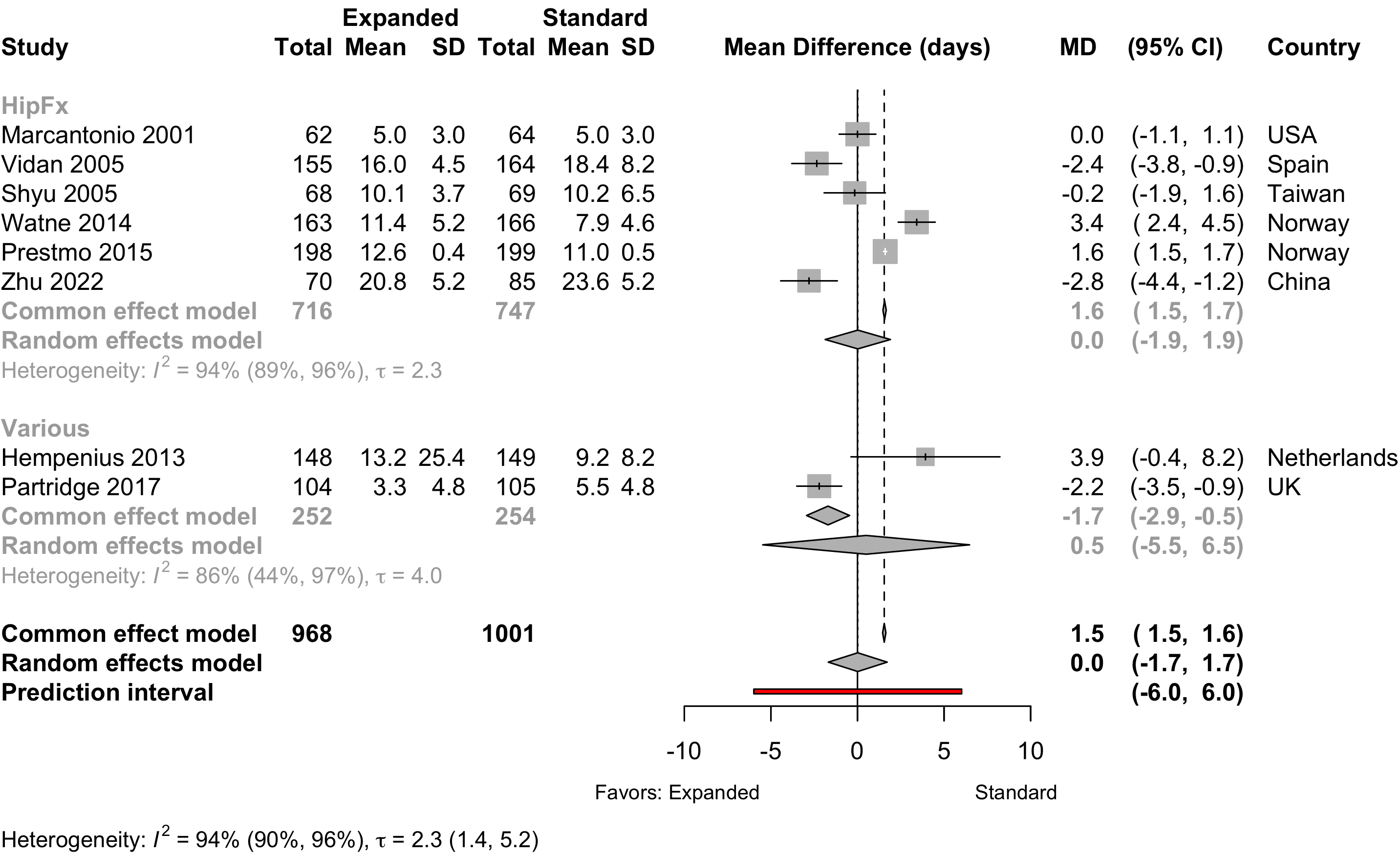
Ommundsen 2018 reported only median and between-group p-value and was excluded.
Nonrandomized
Figure 21. Mean difference in lengths of stay in nonrandomized studies.
Discharge Location
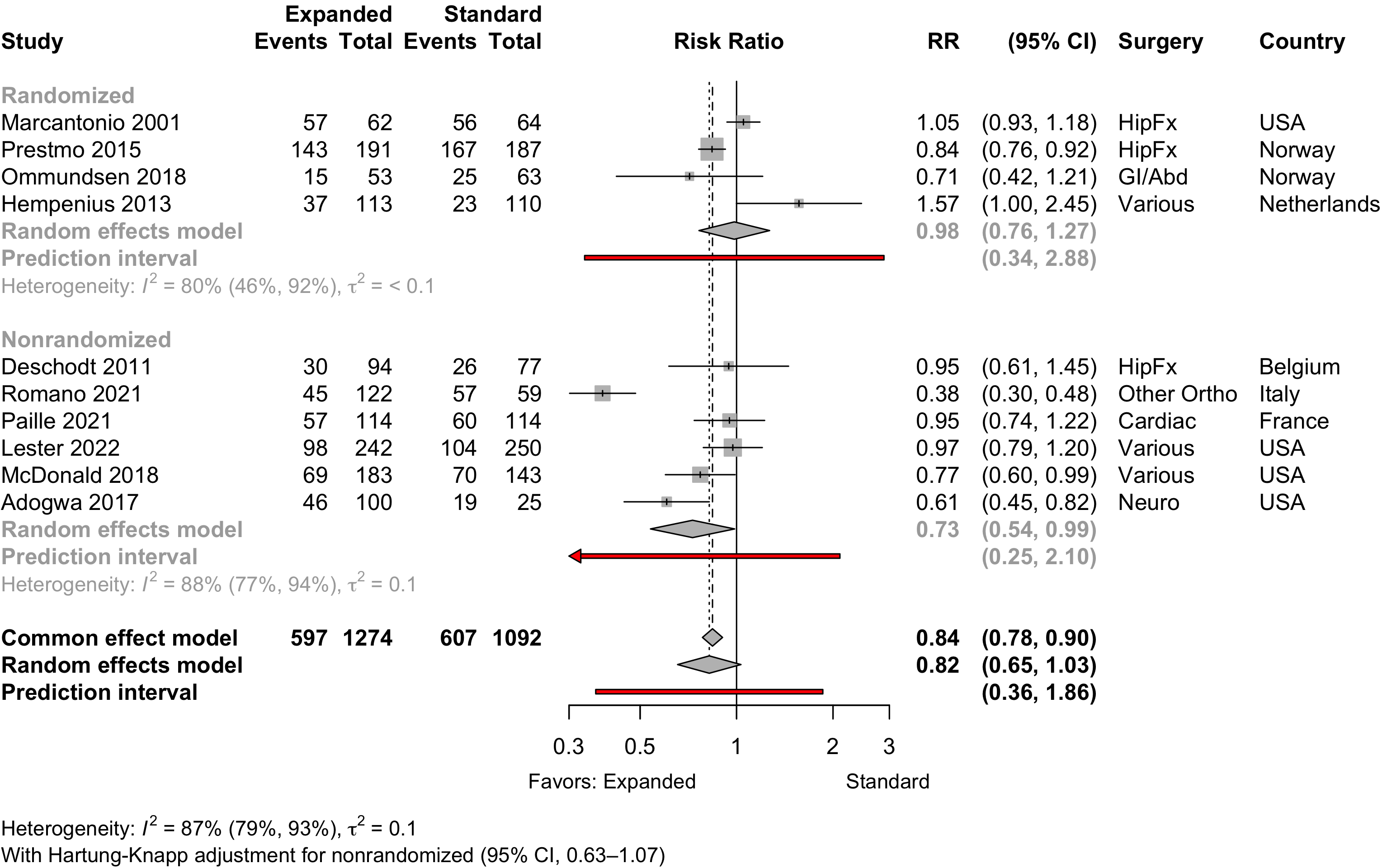
Table 17. Discharge location in studies comparing expanded with standard preoperative assessments.
| Study | N | Arm | Agea | Country | Discharge to Institution | RR (95% CI) | |
|---|---|---|---|---|---|---|---|
| N (%) | 0 — 100% | ||||||
| Randomized Clinical Trial — HipFx | |||||||
| 64 | Std | 80.0 (8.0) |
USA | 56 (87.5) | |||
| 62 | Exp | 78.0 (8.0) |
57 (91.9) | 1.05 (0.93-1.18) | |||
| 187 | Std | 83.2 (6.4) |
Norway | 167 (89.3) | |||
| 191 | Exp | 83.4 (5.4) |
143 (74.9) | 0.84 (0.76-0.92) | |||
| Nonrandomized Trial — HipFx | |||||||
| 77 | Std | 81.1 (7.2) |
Belgium | 26 (33.8) | |||
| 94 | Exp | 80.4 (7.0) |
30 (31.9) | 0.95 (0.61-1.45) | |||
| Before-After/Time Series — Other Ortho | |||||||
| 59 | Std | 73.0 {68-77} |
Italy | 57 (96.6) | |||
| 122 | Exp | 70.0 {64-77} |
45 (36.9) | 0.38 (0.30-0.48) | |||
| Randomized Clinical Trial — GI/Abd | |||||||
| 63 | Std | 78.8 (7.8) |
Norway | 25 (39.7) | |||
| 53 | Exp | 78.2 (7.4) |
15 (28.3) | 0.71 (0.42-1.21) | |||
| Retrospective Cohort — Cardiac | |||||||
| 114 | Std | 81.0 {77-83} |
France | 60 (52.6) | |||
| 114 | Exp | 80.0 {79-82} |
57 (50.0) | 0.95 (0.74-1.22) | |||
| Before-After/Time Series — Neuro | |||||||
| 25 | Std | 73.0 (4.9) |
USA | 19 (76.0) | |||
| 100 | Exp | 73.6 (6.0) |
46 (46.0) | 0.61 (0.45-0.82) | |||
| Before-After/Time Series — Various | |||||||
| 250 | Std | 82.7 {78-87} |
USA | 104 (41.6) | |||
| 242 | Exp | 81.1 {78-86} |
98 (40.5) | 0.97 (0.79-1.20) | |||
| 143 | Std | 71.9 (6.4) |
USA | 70 (49.0) | |||
| 183 | Exp | 75.6 (6.8) |
69 (37.7) | 0.77 (0.60-0.99) | |||
| Randomized Clinical Trial — Various | |||||||
| 110 | Std | 77.6 (7.7) |
Netherlands | 23 (20.9) | |||
| 113 | Exp | 77.5 (6.7) |
37 (32.7) | 1.57 (1.00-2.45) | |||
| Exp: expanded preoperative evaluation; Std: standard preoperative evaluation; RR: risk ratio; Various: more that one procedure category. | |||||||
| a Mean Med (SD)[Range]{IQR}. | |||||||
Pooled
Figure 22. Pooled comparison of discharge location in randomized and nonrandomized designs.
Mortality
Table 18. Reported mortality in randomized clinical trials.
| Study | N | Arm | Surgery | ASA | Agea | Mortality | RD (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| PS | N (%) | 0 - 100% | ||||||
| Hospital | ||||||||
| 164 | Std | HipFx | NR | 82.6 (7.4) |
9 (5.5) | — | ||
| 155 | Exp | 81.1 (7.8) |
1 (0.6) | -4.8% (-8.5, -1.1) | ||||
| 166 | Std | HipFx | NR | 85.0 {46-101} |
3 (1.8) | — | ||
| 163 | Exp | 84.0 {55-99} |
6 (3.7) | 1.9% (-1.7, 5.4) | ||||
| 133 | Std | Various | NR | 77.6 (7.7) |
4 (3.0) | — | ||
| 127 | Exp | 77.5 (6.7) |
10 (7.9) | 4.9% (-0.6, 10.4) | ||||
| 30-day | ||||||||
| 63 | Std | HipFx | NR | 77.7 (7.1) |
0 (0) | — | ||
| 63 | Exp | 77.6 (8.3) |
0 (0) | 0.0% (-3.1, 3.1) | ||||
| 63 | Std | GI/Abd | NR | 78.8 (7.8) |
3 (4.8) | — | ||
| 53 | Exp | 78.2 (7.4) |
2 (3.8) | -1.0% (-8.3, 6.4) | ||||
| 90-day | ||||||||
| 63 | Std | HipFx | NR | 77.7 (7.1) |
1 (1.6) | — | ||
| 63 | Exp | 77.6 (8.3) |
0 (0) | -1.6% (-5.9, 2.7) | ||||
| 63 | Std | GI/Abd | NR | 78.8 (7.8) |
4 (6.3) | — | ||
| 53 | Exp | 78.2 (7.4) |
3 (5.7) | -0.7% (-9.3, 8.0) | ||||
| 133 | Std | Various | NR | 77.4 (7.7) |
9 (6.8) | — | ||
| 127 | Exp | 77.4 (6.9) |
17 (13.4) | 6.6% (-0.7, 13.9) | ||||
| 1-year | ||||||||
| 164 | Std | HipFx | NR | 82.6 (7.4) |
42 (25.6) | — | ||
| 155 | Exp | 81.1 (7.8) |
29 (18.7) | -6.9% (-16.0, 2.2) | ||||
| 62 | Std | HipFx | NR | 78.9 (7.3) |
15 (24.2) | — | ||
| 60 | Exp | 77.4 (8.2) |
13 (21.7) | -2.5% (-17.4, 12.4) | ||||
| ASA PS: American Society of Anesthesiologists Physical Status; Exp: expanded; Std: standard; NR: not reported; RD: risk difference.; GI: gastrointestinal; Abd: abdominal (includes hepatic); Various: more that four procedures. | ||||||||
| a Mean Med (SD)[Range]{IQR}. | ||||||||
Table 19. Reported mortality in nonrandomized designs (all retrospective cohort studies).
| Study | N | Arm | Surgery | ASA | Agea | Mortality | RD (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| PS | N (%) | 0 - 100% | ||||||
| Hospital | ||||||||
| 77 | Std | HipFx | NR | 81.1 (7.2) |
0 (0) | — | ||
| 94 | Exp | 80.4 (7.0) |
1 (1.1) | 1.1% (-2.0, 4.1) | ||||
| 107 | Std | GI/Abd | 1234 | 75.3 (5.1) |
4 (3.7) | — | ||
| 203 | Exp | 77.5 (4.8) |
8 (3.9) | 0.2% (-4.3, 4.7) | ||||
| 54 | Std | GI/Abd | NR | 75.0 {70-81} |
2 (3.7) | — | ||
| 18 | Exp | 80.0 {72-83} |
0 (0) | -3.7% (-12.8, 5.4) | ||||
| 114 | Std | Cardiac | NR | 81.0 {77-83} |
7 (6.1) | — | ||
| 114 | Exp | 80.0 {79-82} |
3 (2.6) | -3.5% (-8.8, 1.8) | ||||
| 143 | Std | Various | NR | 71.9 (6.4) |
0 (0) | — | ||
| 183 | Exp | 75.6 (6.8) |
1 (0.5) | 0.5% (-1.1, 2.2) | ||||
| 30-day | ||||||||
| 132 | Std | HipFx | 1234 | 82.0 (7.6) |
6 (4.5) | — | ||
| 131 | Exp | 81.1 (7.5) |
5 (3.8) | -0.7% (-5.6, 4.1) | ||||
| 54 | Std | Other Ortho | NR | 75.0 (6.1) |
1 (1.9) | — | ||
| 54 | Exp | 74.1 (6.2) |
0 (0) | -1.9% (-6.8, 3.1) | ||||
| 222 | Std | GI/Abd | NR | 77.0 {73-81} |
17 (7.7) | — | ||
| 221 | Exp | 77.0 {74-82} |
14 (6.3) | -1.3% (-6.1, 3.4) | ||||
| 63 | Std | GI/Abd | 1234 | 81.4 {74-89} |
2 (3.2) | — | ||
| 86 | Exp | 80.6 {74-87} |
2 (2.3) | -0.8% (-6.2, 4.5) | ||||
| 107 | Std | GI/Abd | 1234 | 75.3 (5.1) |
5 (4.7) | — | ||
| 203 | Exp | 77.5 (4.8) |
9 (4.4) | -0.2% (-5.1, 4.7) | ||||
| 117 | Std | GI/Abd | NR | 82.0 {78-85} |
9 (7.7) | — | ||
| 90 | Exp | 79.0 {76-83} |
3 (3.3) | -4.4% (-10.4, 1.7) | ||||
| 160 | Std | Various | NR | 68.3 (11.2) |
51 (31.9) | — | ||
| 150 | Exp | 71.3 (10.6) |
32 (21.3) | -10.5% (-20.3, -0.8) | ||||
| 5,275 | Std | Various | 1234 | 60.3 (13.4) |
84 (1.6) | — | ||
| 3,878 | Exp | 60.3 (13.7) |
26 (0.7) | -0.9% (-1.3, -0.5) | ||||
| 308 | Std | Various | 234 | 80.0 (5.0) |
12 (3.9) | — | ||
| 158 | Exp | 80.0 (5.0) |
4 (2.5) | -1.4% (-4.6, 1.9) | ||||
| 250 | Std | Various | NR | 82.7 {78-87} |
6 (2.4) | — | ||
| 242 | Exp | 81.1 {78-86} |
10 (4.1) | 1.7% (-1.4, 4.9) | ||||
| 101 | Std | Urol | NR | 3 (3.0) | — | |||
| 124 | Exp | 0 (0) | -3.0% (-6.7, 0.7) | |||||
| 25 | Std | Neuro | NR | 73.0 (4.9) |
0 (0) | — | ||
| 100 | Exp | 73.6 (6.0) |
0 (0) | 0.0% (-5.5, 5.5) | ||||
| 90-day | ||||||||
| 117 | Std | GI/Abd | NR | 82.0 {78-85} |
12 (10.3) | — | ||
| 90 | Exp | 79.0 {76-83} |
9 (10.0) | -0.3% (-8.5, 8.0) | ||||
| 1-year | ||||||||
| 63 | Std | GI/Abd | 1234 | 81.4 {74-89} |
7 (11.1) | — | ||
| 86 | Exp | 80.6 {74-87} |
3 (3.5) | -7.6% (-16.3, 1.1) | ||||
| 107 | Std | GI/Abd | 1234 | 75.3 (5.1) |
10 (9.3) | — | ||
| 203 | Exp | 77.5 (4.8) |
23 (11.3) | 2.0% (-5.0, 9.0) | ||||
| 117 | Std | GI/Abd | NR | 82.0 {78-85} |
16 (13.7) | — | ||
| 90 | Exp | 79.0 {76-83} |
17 (18.9) | 5.2% (-5.0, 15.4) | ||||
| 160 | Std | Various | NR | 68.3 (11.2) |
126 (78.8) | — | ||
| 150 | Exp | 71.3 (10.6) |
99 (66.0) | -12.7% (-22.6, -2.9) | ||||
| 5,275 | Std | Various | 1234 | 60.3 (13.4) |
320 (6.1) | — | ||
| 2,781 | Exp | 60.3 (13.7) |
78 (2.8) | -3.3% (-4.2, -2.4) | ||||
| ASA PS: American Society of Anesthesiologists Physical Status; RD: risk difference; GI: gastrointestinal; Abd: abdominal (includes hepatic); Various: more that one procedure category. | ||||||||
| a Mean Med (SD)[Range]{IQR}. | ||||||||
Pooled
Randomized
Figure 23. Risk ratio for hospital or 30-day mortality in randomized clinical trials.
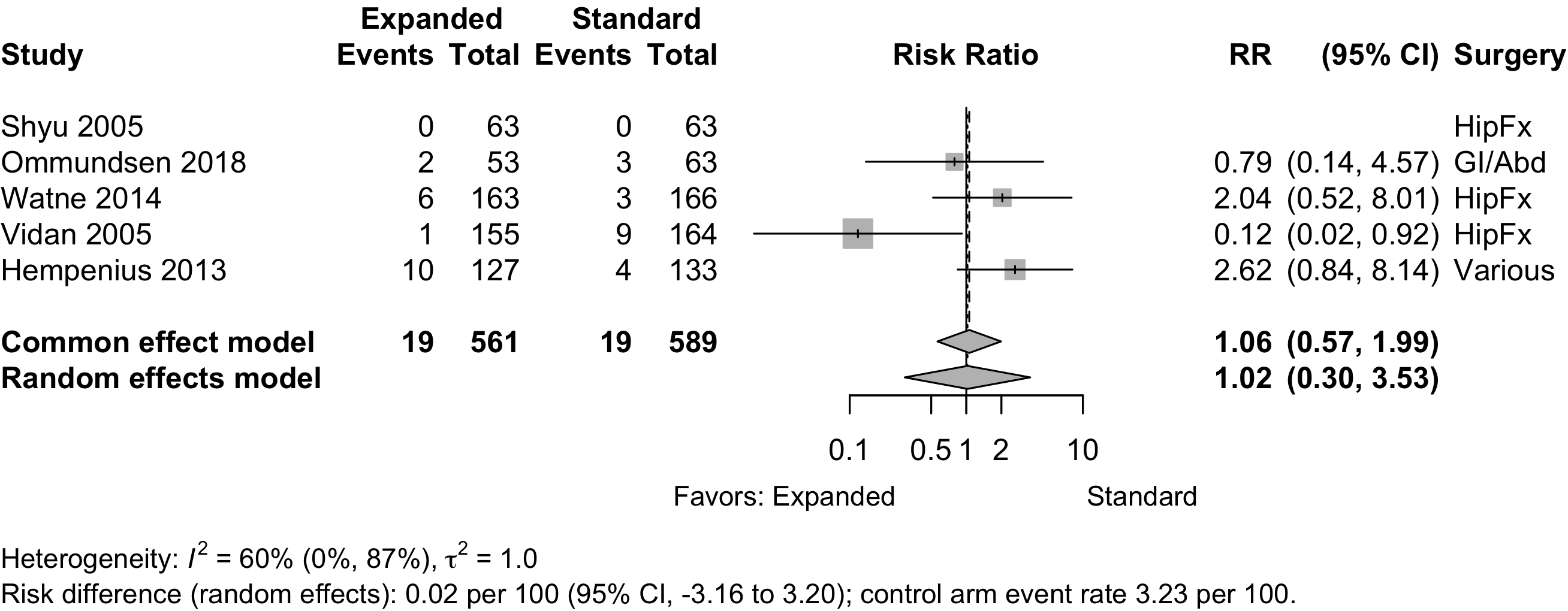
Figure 24. Risk ratio for 1-year mortality in randomized clinical trials.
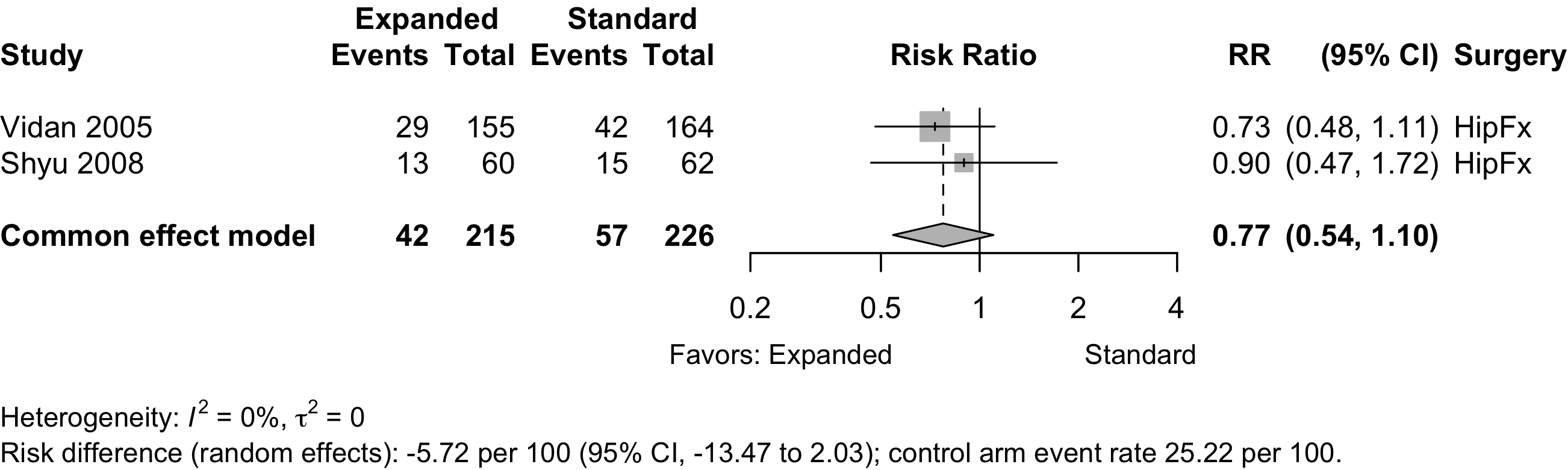
Nonrandomized
Figure 25. Risk ratio for hospital or 30-day mortality in nonrandomized studies.
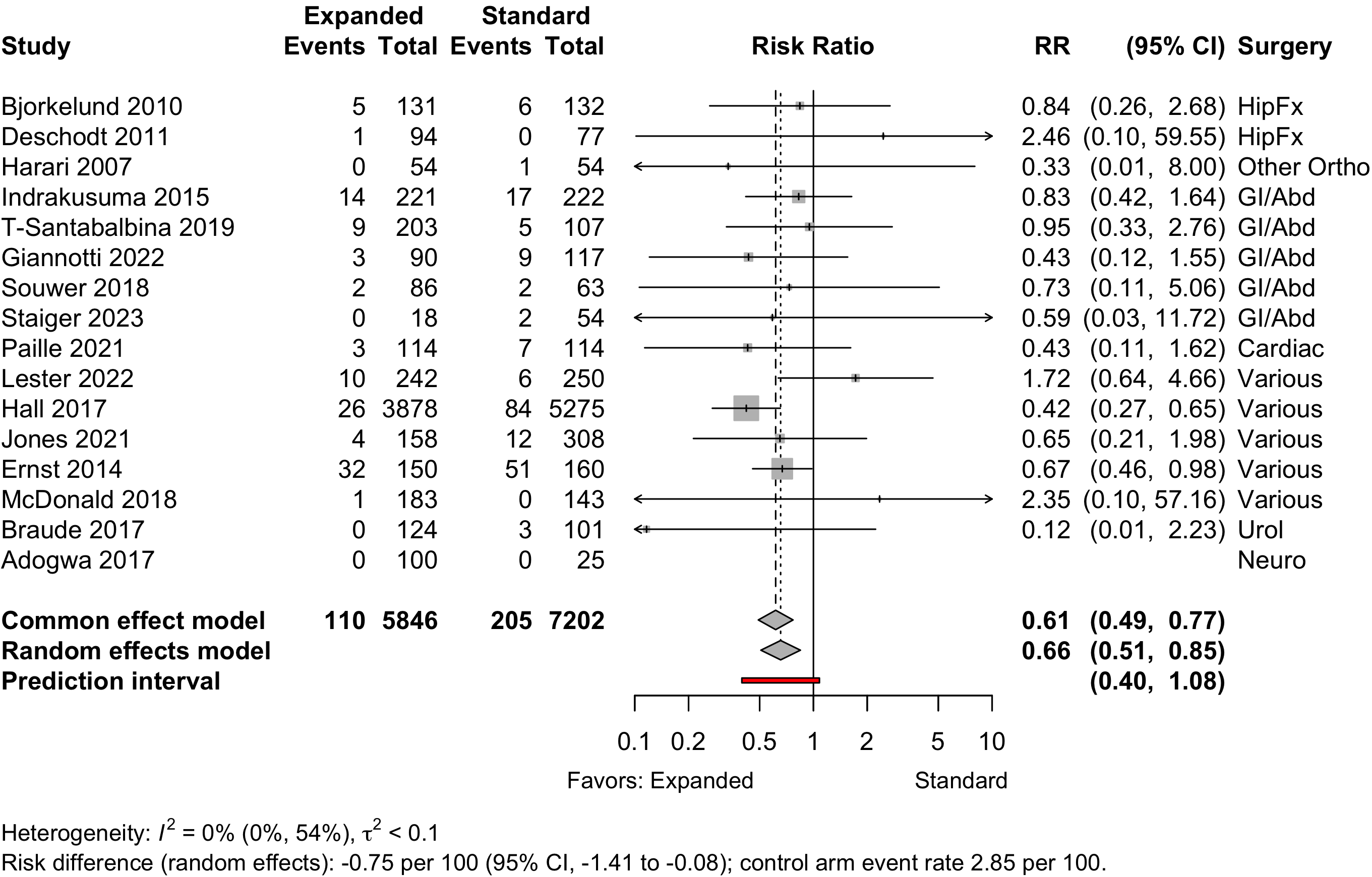
Figure 26. Risk ratio for 1-year mortality in nonrandomized studies.
Risk of Bias
Randomized
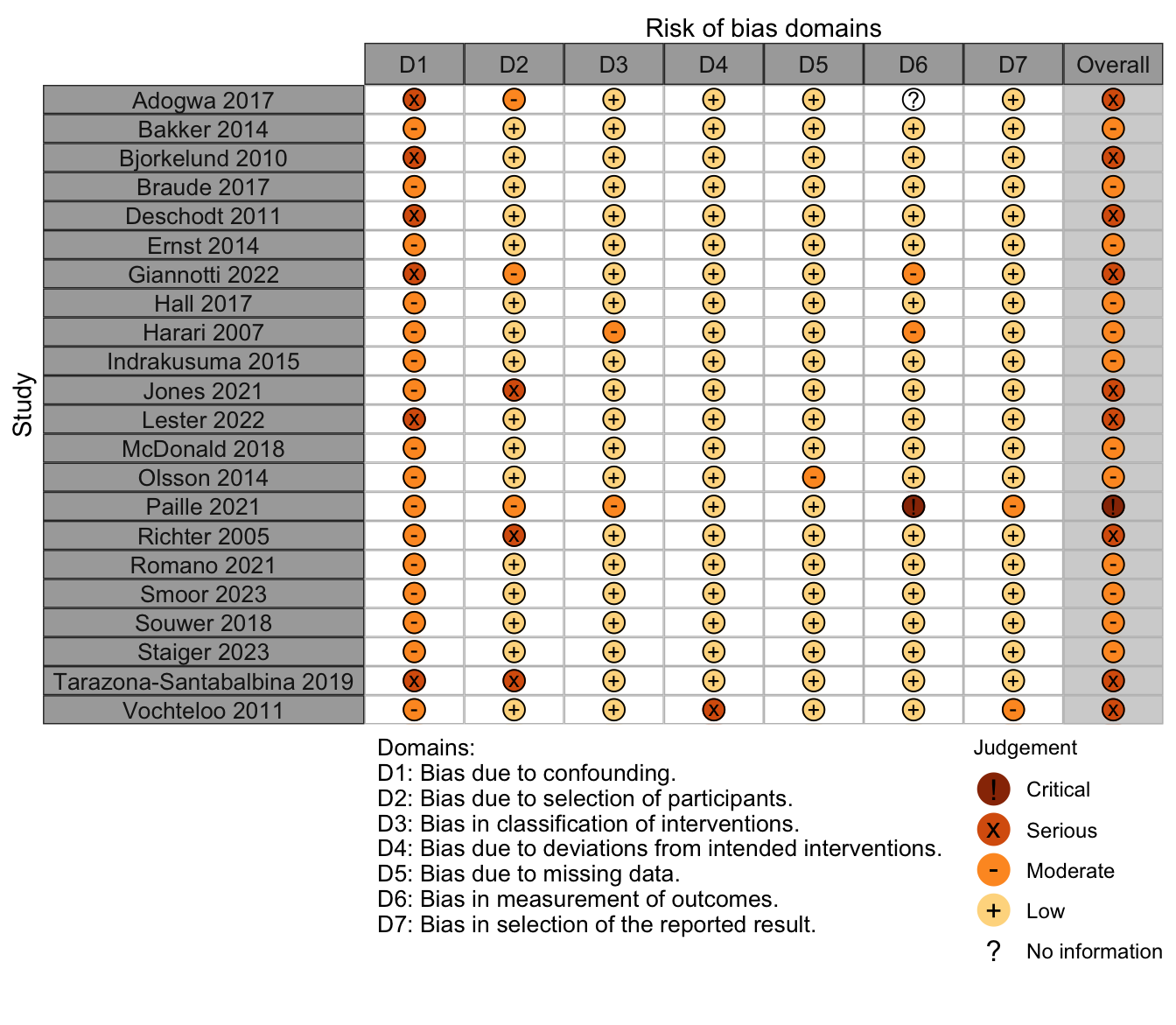
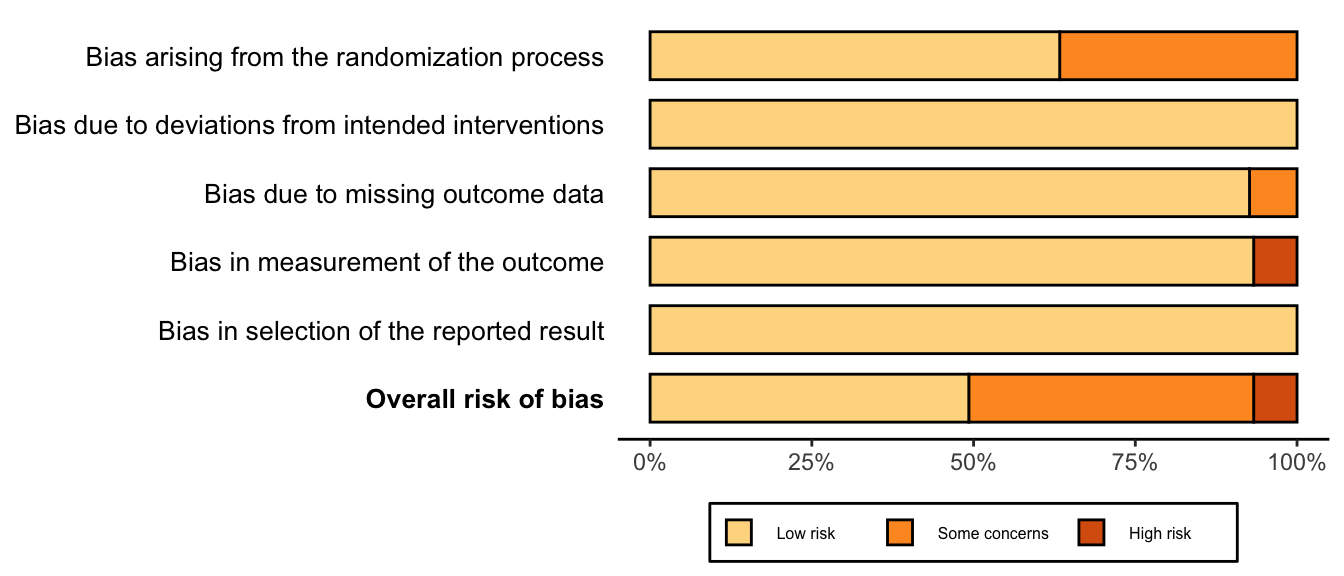
Figure 27. Summary risk of bias assessment for randomized clinical trials (expanded preoperative evaluation).
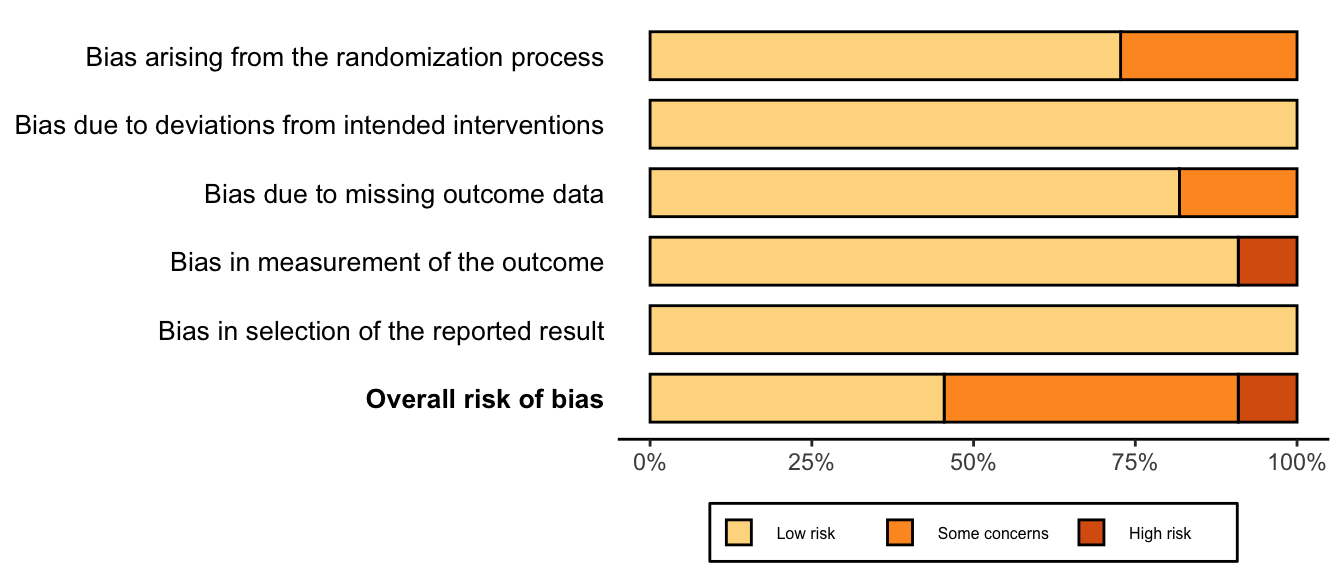
Figure 28. Risk of bias assessments for randomized clinical trials (expanded preoperative evaluation).
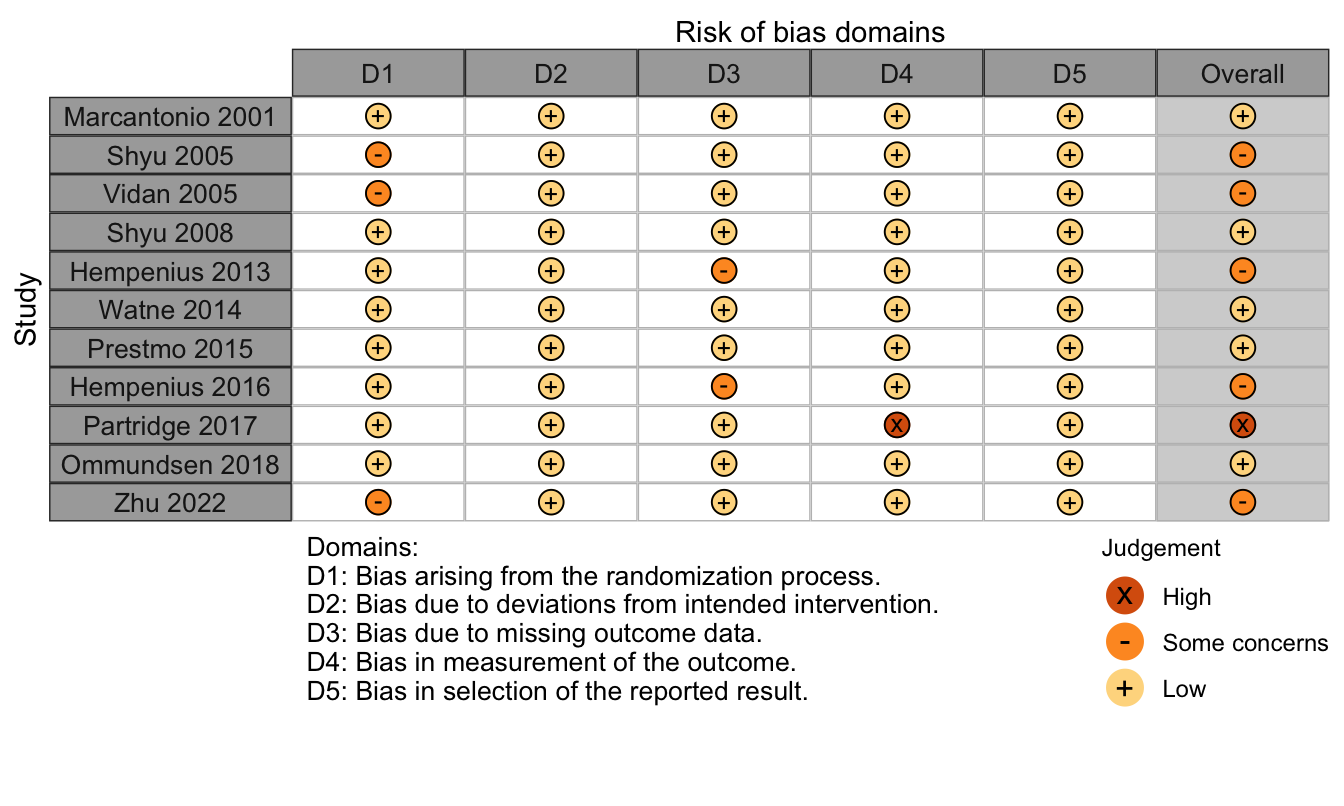
Nonrandomized
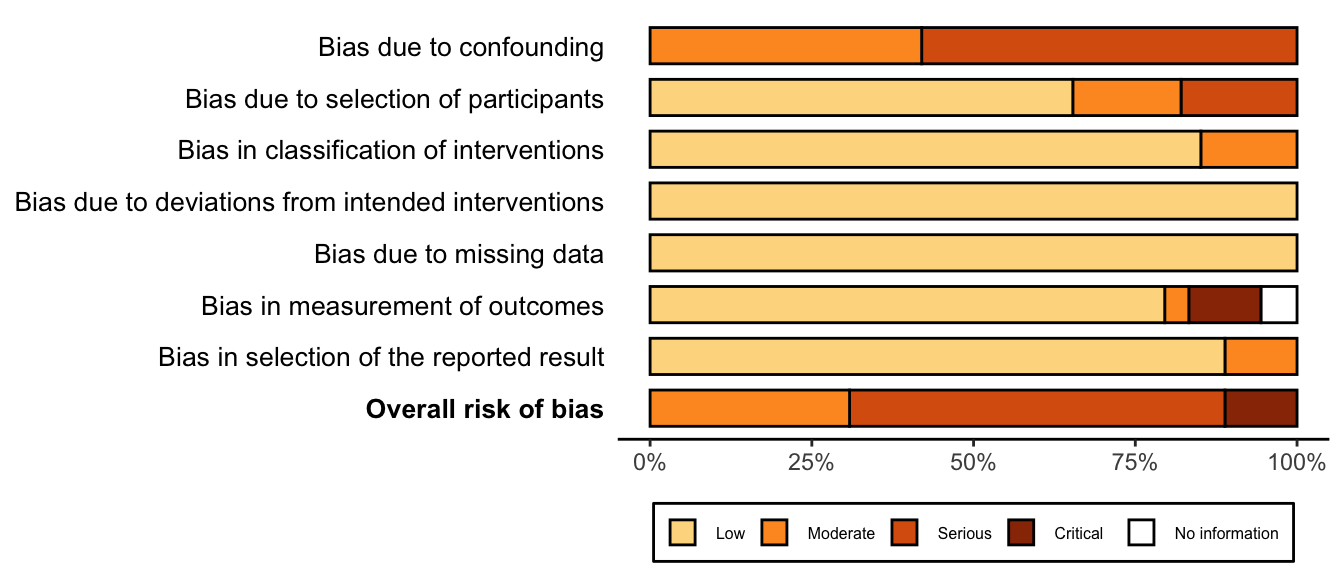
Figure 29. Summary risk of bias assessment for nonrandomized studies (expanded preoperative evaluation).
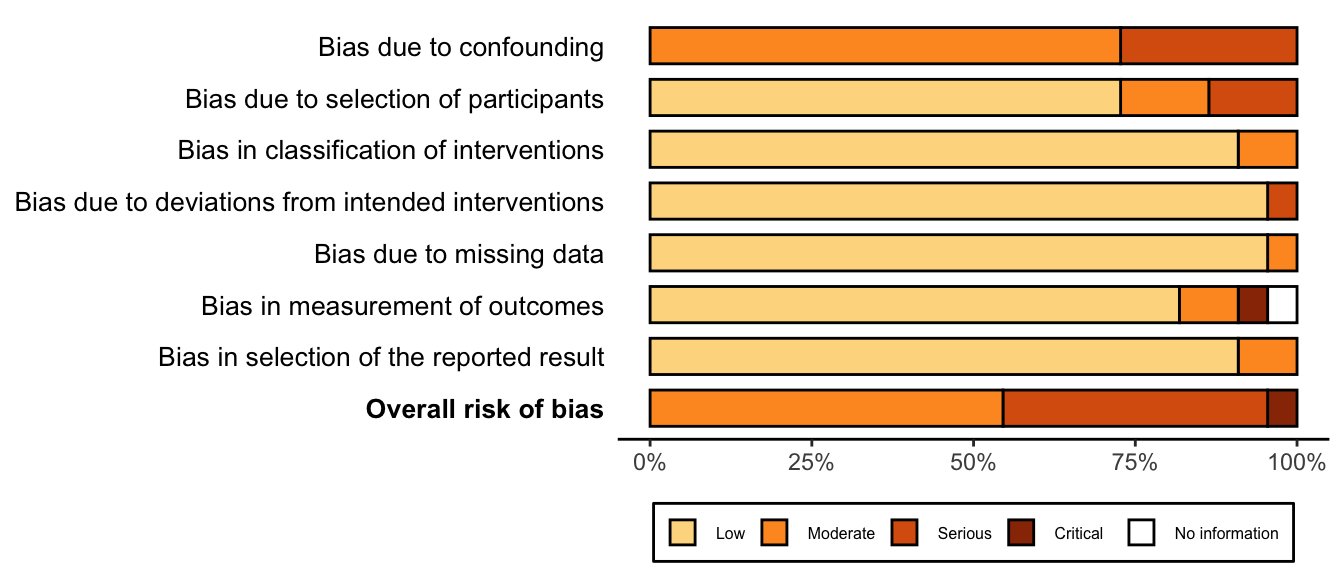
Figure 30. Risk of bias assessments for nonrandomized studies (expanded preoperative evaluation).