| Outcome | RCT | NRSI* | TIVA | Inhaled | GRADE† | Effect | Estimate (95% CI) |
|---|---|---|---|---|---|---|---|
| N (Total) | N (Total) | ||||||
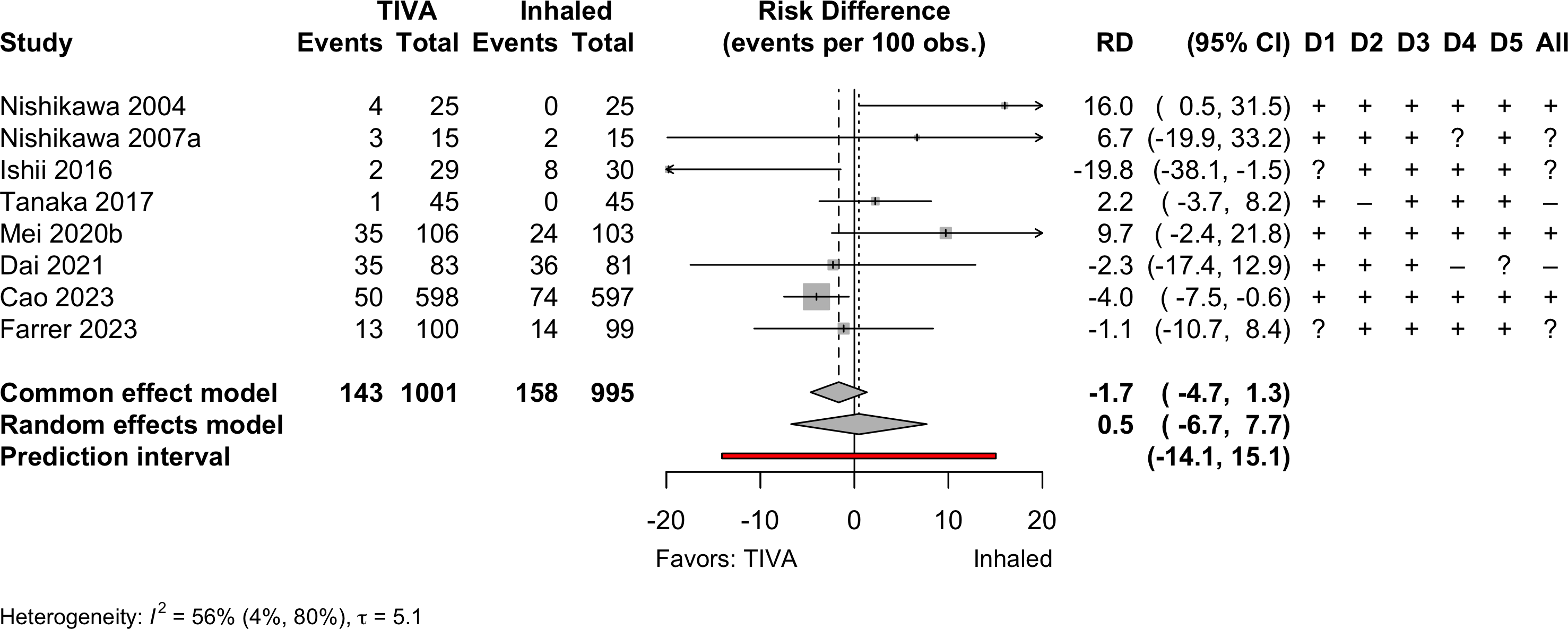
| Delirium | 8 | 143 (1,001) | 158 (995) | RR | 0.94 (0.62–1.43) | ||
| 5 | 10,297 (142,850) | 32,955 (427,929) | OR | 1.01 (0.39–2.63) | |||
| Neurocognitive disorder <30 days | 5 | 125 (704) | 175 (703) | RR | 0.72 (0.54–0.96) | ||
| 1 | 24 (160) | 24 (119) | RR | 0.74 (0.44–1.24) | |||
| Neurocognitive disorder days to 1 yr | 1 | 4 (96) | 6 (97) | RR | 0.67 (0.20–2.31) | ||
| 3 | 40 (252) | 32 (207) | RR | 1.09 (0.72–1.66) | |||
| Physical function | none | none | |||||
| Complications | 10 | 9 | see below | ||||
| Patient satisfaction | 3 | 90 (109) | 82 (141) | RR | 1.39 (1.19–1.63)‡ | ||
| Length of stay (days) | 6 | (1,343) | (1,341) | MD | 0.0 (-1.5 to 1.4) | ||
| 4 | (147,809) | (432,893) | MD | -0.6 (-2.4 to 1.5) | |||
| Discharged to institution | 1 | 8 (9) | 26 (20) | RR | 1.46 (0.69–3.41) | ||
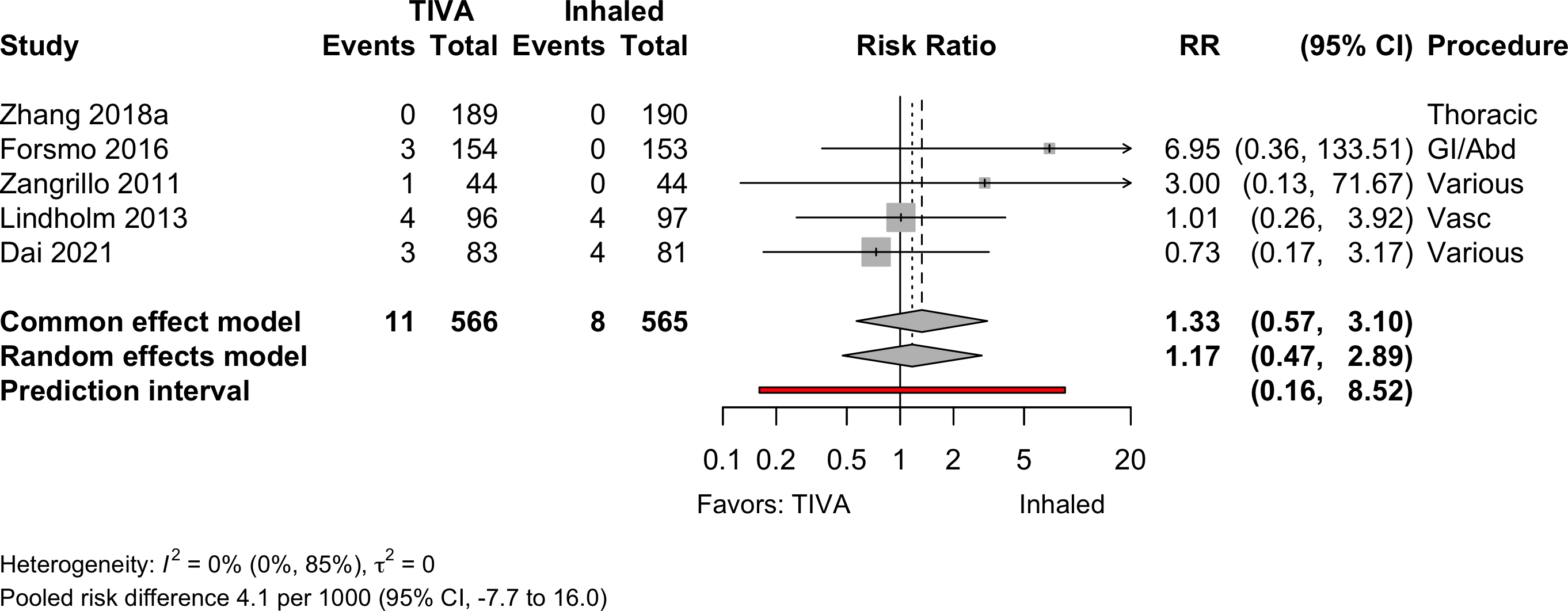
| Mortality (in-hospital and 30-day) | 4 | 11 (377) | 8 (375) | RR | 1.17 (0.47–2.89) | ||
| 5 | 11 (566) | 8 (565) | RD/1000 | 4.1 (-7.7 to 16.0) | |||
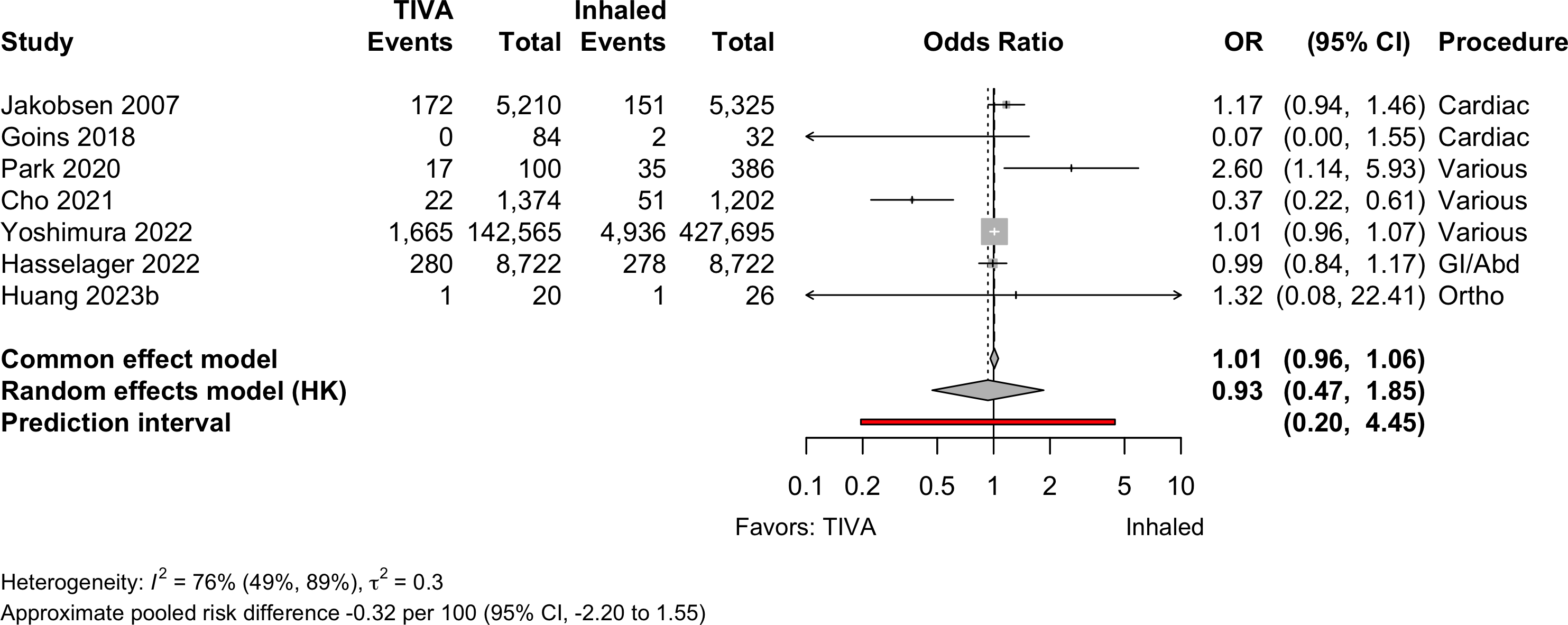
| 7 | 1,876 (149,333) | 5,175 (434,640) | OR | 0.93 (0.47–1.85) | |||
| RD/1000 | -0.32 (-2.20 to 1.55) | ||||||
| Mortality (1-year) | 1 | 1 | 5 (64) | 4 (70) | RR | 1.47 (0.42–5.18) | |
| RD/1000 | 24.2 (-46.5 to 95.0) | ||||||
| RCT: randomized clinical trial; NRSI: nonrandomized studies of interventions; GRADE: Grades of Recommendation, Assessment, Development, and Evaluation; RR: risk ratio; MD: mean difference; RD: risk difference. | |||||||
| * Results from nonrandomized designed shown only when evidence not available from randomized trials. | |||||||
| † Very low: ⨁◯◯◯; Low: ⨁⨁◯◯; Moderate: ⨁⨁⨁◯; High: ⨁⨁⨁⨁. | |||||||
| ‡ Comparing higher/highest category or categories with lower ones. | |||||||
TIVA versus Inhaled (volatile) Anesthesia
Key Question
Among older patients undergoing surgery with general anesthesia, does the use of intravenous agents for maintenance of anesthesia improve postoperative outcomes compared with inhaled agents?
Balance Tables
| Outcome | RCT | NRSI | TIVA | Inhaled | GRADE* | Effect | Estimate (95% CI) |
|---|---|---|---|---|---|---|---|
| N (Total) | N (Total) | ||||||
| Myocardial infarction | 1 | 5 | 4,027 (157,987) | 8,898 (443,067) | OR | 0.90 (0.85–0.96) | |
| 2 | 5 | 4,027 (158,031) | 8,898 (443,111) | RD/1000 | -1.9 (3.0 to -0.7)† | ||
| Cardiac arrest | 1 | 1 | 5 (1,972) | 1 (1,799) | RR | 3.47 (0.57–21.2)‡ | |
| RD/1000 | 1.7 (-0.7 to 4.1)‡ | ||||||
| Bradycardia | 4 | 1 | 20 (416) | 16 (416) | RR | 1.57 (0.42–5.81) | |
| RD/1000 | 13.4 (-43.1 to 69.9) | ||||||
| Hypotension | 2 | 2 | 226 (919) | 237 (884) | RR | 0.99 (0.91–1.07 | |
| RD/1000 | -24.6 (-58.6 to 9.4) | ||||||
| Stroke | 1 | 1 | 37 (9,320) | 42 (9,319) | OR | 1.14 (0.73–1.78) | |
| RD/1000 | 0.5 (-2.4 to 1.3)† | ||||||
| Acute kidney injury | 1 | 5 | 823 (144,819) | 2,283 (430,321) | OR | 0.99 (0.88–1.11) | |
| RD/1000 | -0.2 (-4.4 to 4.1)† | ||||||
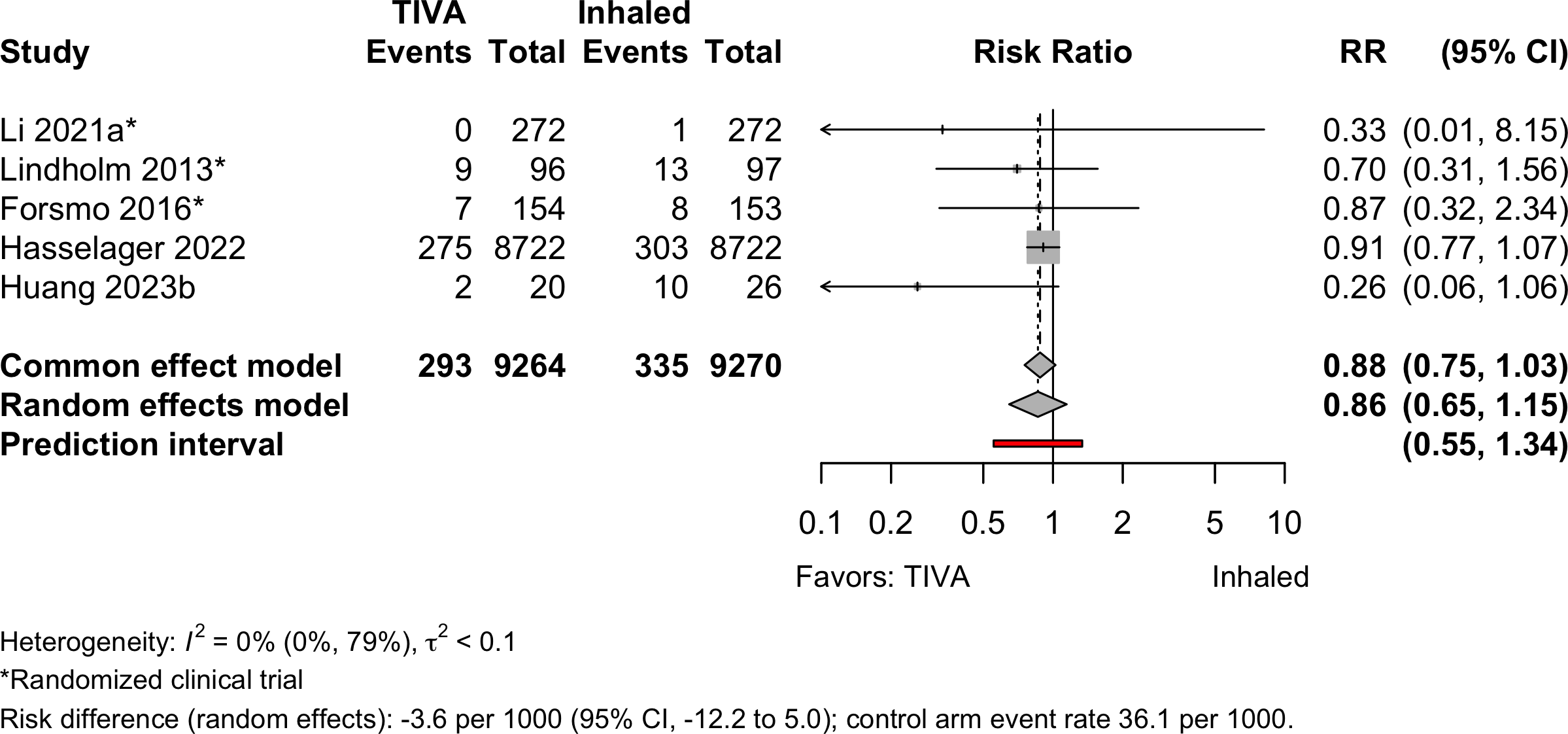
| Pneumonia | 3 | 2 | 293 (9,264) | 335 (9,270) | OR | 0.80 (0.39–1.64) | |
| RD/1000 | -3.6 (-8.3 to 1.2)† | ||||||
| Pulmonary edema | 2 | 0 (143,939) | 3 (428,897) | — | —§ | ||
| Pulmonary embolism | 2 | 3 | 212 (153,413) | 465 (438,369) | OR | 1.32 (1.13–1.53) | |
| RD/1000 | 0.2 (-2.0 to 2.4)† | ||||||
| Respiratory failure | 2 | 2 | 670 (151,981) | 1,718 (437,111) | OR | 0.87 (0.79–0.95) | |
| RD/1000 | 0.0 (-2.0 to 2.0)† | ||||||
| RCT: randomized clinical trial; NRSI: nonrandomized studies of interventions; GRADE: Grades of Recommendation, Assessment, Development, and Evaluation; RR: risk ratio; OR: odds ratio; RD: risk difference. | |||||||
| * Very low: ⨁◯◯◯; Low: ⨁⨁◯◯; Moderate: ⨁⨁⨁◯; High: ⨁⨁⨁⨁. | |||||||
| † Approximate owing to pruning in studies using propensity matching. | |||||||
| ‡ Common effects model. | |||||||
| § No events in 1 study; 3 in the other. | |||||||
Outcomes Reported
| Outcome | RCT, N = 34 | NR Trial, N = 1 | Prosp Coh, N = 3 | Retro Coh, N = 13 |
|---|---|---|---|---|
| ADL | — | — | — | — |
| Complications | 10 (29%) | 1 (100%) | — | 11 (85%) |
| DNCR/POCD | 9 (26%) | 1 (100%) | 2 (67%) | 1 (7.7%) |
| Delirium | 8 (24%) | — | 1 (33%) | 4 (31%) |
| Delirium duration | — | — | — | — |
| Discharge location | — | — | — | 1 (7.7%) |
| Mortality | 5 (15%) | — | — | 7 (54%) |
| Opioid use | 2 (5.9%) | — | — | — |
| Pain | 1 (2.9%) | — | — | — |
| QoR | — | — | — | — |
| Readmission | 1 (2.9%) | — | — | — |
| Satisfaction | 3 (8.8%) | — | — | — |
| ADL: activities of daily living; NCR: neurocognitive recovery; POCD: postoperative neurocognitive disorder; QoR: quality of recovery; RCT: randomized clinical trial; NR Trial: non-randomized trial; Prosp Coh: prospective cohort; Retro Coh: retrospective cohort. | ||||
| Outcome | RCT, N = 34 | NR Trial, N = 1 | Prosp Coh, N = 3 | Retro Coh, N = 13 |
|---|---|---|---|---|
| Delirium duration | 1 (2.9%) | — | — | — |
| Length of stay | 6 (18%) | — | — | 4 (31%) |
| Opioid use | 3 (8.8%) | — | — | — |
| RCT: randomized clinical trial; NR Trial: non-randomized trial; Prosp Coh: prospective cohort; Retro Coh: retrospective cohort. | ||||
| Outcome | RCT, N = 34 | NR Trial, N = 1 | Prosp Coh, N = 3 | Retro Coh, N = 13 |
|---|---|---|---|---|
| ADL | — | — | — | — |
| DNCR/POCD | 16 (47%) | 1 (100%) | — | — |
| Delirium | 3 (8.8%) | — | — | — |
| Complications | — | — | — | — |
| Pain | 4 (12%) | — | — | — |
| Quality of life | — | — | — | — |
| QoR | 1 (2.9%) | — | — | — |
| Satisfaction | — | — | — | — |
| ADL: activities of daily living; NCR: neurocognitive recovery; POCD: postoperative neurocognitive disorder; QoR: quality of recovery; RCT: randomized clinical trial; NR Trial: non-randomized trial; Prosp Coh: prospective cohort; Retro Coh: retrospective cohort. | ||||
Included Studies
See Appendix for detailed summary study and patient characteristics including primary outcomes.
| Design | Studies |
|---|---|
| Randomized Clinical Trial | 34 |
| Nonrandomized Trial | 1 |
| Prospective Cohort | 3 |
| Retrospective Cohort | 13 |
| Total | 51 |
Design, centers, country, and surgery
| ID | Study | Centers | Enrolled | Countrya | Surgery |
|---|---|---|---|---|---|
| Randomized Clinical Trial | |||||
| 20083 | 1 | 50 | Greece | Cardiac | |
| 18510 | 1 | 102 | South Korea | Cardiac | |
| 13719 | 1 | 88 | Turkey | Cardiac | |
| 4033 | 1 | 90 | Chinaa | ENT | |
| 16931 | 1 | 69 | Chinaa | ENT | |
| 15440 | 1 | 60 | Irana | GI/Abdominal | |
| 11652 | 1 | 653 | Norway | GI/Abdominal | |
| 16559 | 1 | 150 | Chinaa | GI/Abdominal | |
| 16572 | 1 | 59 | Japan | GI/Abdominal | |
| 17 | 4 | 544 | Chinaa | GI/Abdominal | |
| 385 | 1 | 50 | Japan | GI/Abdominal | |
| 331 | 1 | 30 | Japan | GI/Abdominal | |
| 16622 | 1 | 220 | Chinaa | GI/Abdominal | |
| 18152 | 1 | 80 | Chinaa | GI/Abdominal | |
| 9011 | 1 | 180 | Chinaa | GI/Abdominal|Ortho|ENT | |
| 2794 | 1 | 45 | Ireland | Gyn|Urol | |
| 5915 | 1 | 60 | USA | Headneck | |
| 16557 | 1 | 124 | Germany | Ophtho | |
| 16587 | 1 | 96 | Germany | Ophtho | |
| 18987 | 1 | 240 | Chinaa | Ortho | |
| 285 | 1 | 100 | USA | Ortho | |
| 18378 | 2 | 200 | USA | Ortho|Spine | |
| 16726 | 1 | 224 | Chinaa | Spine | |
| 16556 | 1 | 148 | Japan | Thoracic | |
| 150 | 1 | 104 | Chinaa | Thoracic | |
| 20412 | 1 | 62 | Chinaa | Thoracic | |
| 10 | 1 | 392 | Chinaa | Thoracic | |
| 376 | 1 | 153 | Italy | Thoracic|Vasc | |
| 16547 | 1 | 100 | Turkey | Urol | |
| 534 | 1 | 2,216 | Chinaa | Variousb | |
| 17101 | 14 | 1,228 | Chinaa | Variousb | |
| 6506 | 1 | 164 | Chinaa | Variousb | |
| 18867 | 1 | 140 | USA | Variousb | |
| 447 | 1 | 231 | Norway | Vasc | |
| Nonrandomized Trial | |||||
| 13116 | 1 | 265 | Chinaa | Thoracic | |
| Prospective Cohort | |||||
| 17080 | 1 | 76 | USA | General|Spine|Thoracic|Urol | |
| 155 | 1 | 105 | USA | General|Thoracic|Urol | |
| 22 | 1 | 300 | Japan | Ortho | |
| Retrospective Cohort | |||||
| 332 | 1 | 116 | USA | Cardiac | |
| 234 | 3 | 10,535 | Denmark | Cardiac | |
| 20764 | 1 | 109 | Japan | Cardiac | |
| 17551 | 22,179 | Denmark | GI/Abdominal | ||
| 15331 | 1 | 1,934 | South Africa | GI/Abdominal | |
| 14821 | 1 | 265 | Japan | GI/Abdominal | |
| 18477 | 1 | 46 | Taiwan | Ortho | |
| 383 | c | 21,899 | Japan | Ortho | |
| 18986 | 1 | 281 | South Korea | Spine | |
| 132 | 1 | 3,084 | South Korea | Thoracic | |
| 6964 | 1 | 3,045 | South Korea | Variousb | |
| 1134 | 1 | 1,254 | South Korea | Variousb | |
| 16890 | 1,730 | 738,600 | Japan | Variousb | |
| GI: gastrointestinal; Ortho: orthopedic; Ent: ear, nose, and throat; Neuro: neurological; Oralmax: oral maxillofacial; Vasc: vascular. | |||||
| a Non very-high Human Development Index country. | |||||
| b Described as various or more than 4 different types of surgery. | |||||
| c National national administrative claims database. | |||||
Country Summary
| N = 51a | |
|---|---|
| Country | |
| China | 16 (31%) |
| Japan | 9 (18%) |
| USA | 7 (14%) |
| South Korea | 5 (9.8%) |
| Denmark | 2 (3.9%) |
| Germany | 2 (3.9%) |
| Norway | 2 (3.9%) |
| Turkey | 2 (3.9%) |
| Greece | 1 (2.0%) |
| Iran | 1 (2.0%) |
| Ireland | 1 (2.0%) |
| Italy | 1 (2.0%) |
| South Africa | 1 (2.0%) |
| Taiwan | 1 (2.0%) |
| a n (%) | |
Comparators
Randomized
| Study | N | Arm | ASA | Ageb | MMSEb | Inhaled | TIVA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSa | Des | Iso | Sev | Fen | Pro | Rem | Suf | Oth | |||||
| Gastrointestinal/Abdominal | |||||||||||||
| 25 | Inhaled | 12 | 71.0 (7.0) |
✓ | |||||||||
| 25 | TIVA | 71.0 (8.0) |
● | ||||||||||
| 15 | Inhaled | 12 | 70.9 (6.5) |
✓ | |||||||||
| 15 | TIVA | 71.2 (5.3) |
●c | ||||||||||
| 99 | Inhaled | 70.0 (4.3) |
25.0 (1.7) |
✓ | ○ | ||||||||
| 101 | TIVA | 69.6 (4.8) |
24.6 (1.7) |
● | |||||||||
| 153 | Inhaled | 123 | 66.0 [19-93] |
✓d | ✓d | ○ | ○ | ||||||
| 154 | TIVA | 65.0 [23-89] |
● | ● | |||||||||
| 30 | Inhaled | 76.5 (4.5) |
✓ | ||||||||||
| 29 | TIVA | 77.3 (4.6) |
● | ||||||||||
| 30 | Inhaled | 71.0 (2.6) |
27.3 (1.9) |
✓ | |||||||||
| 30 | TIVA | 73.0 (3.2) |
27.2 (2.4) |
● | |||||||||
| 50 | Inhaled | 23 | 28.9 (1.4) |
✓ | |||||||||
| 50 | Inhaled | 29.4 (1.9) |
✓ | ||||||||||
| 50 | TIVA | 29.2 (1.5) |
● | ||||||||||
| 272 | Inhaled | 65.0 {62-69} |
29 {28-30} |
✓ | ○ | ||||||||
| 272 | TIVA | 64.0 {62-68} |
29 {28-30} |
● | ● | ||||||||
| 40 | Inhaled | 12 | 71.8 (2.1) |
29.7 (0.4) |
✓ | ||||||||
| 40 | TIVA | 72.0 (3.1) |
29.6 (0.4) |
● | |||||||||
| Various | |||||||||||||
| 90 | Inhaled | 12 | 71.3 (5.6) |
27.0 (2.7) |
✓ | ||||||||
| 90 | TIVA | 70.2 (4.3) |
26.0 (2.5) |
● | |||||||||
| 15 | Inhaled | 73.8 [67-86] |
28.0 [25-30] |
✓ | ○ | ||||||||
| 15 | TIVA | 72.9 [65-83] |
27.0 [25-30] |
● | ● | ||||||||
| 99 | Inhaled | 73.6 (5.4) |
✓ | ||||||||||
| 100 | TIVA | 72.9 (5.4) |
● | ● | |||||||||
| 44 | Inhaled | 1234 | 65.0 (11.8) |
✓ | ○ | ||||||||
| 44 | TIVA | 64.0 (12.2) |
● | ||||||||||
| 1,000 | Inhaled | 69.3 (5.1) |
27.4 (1.1) |
✓ | ○ | ○ | |||||||
| 1,000 | TIVA | 71.2 (3.8) |
27.2 (1.1) |
● | ● | ||||||||
| 81 | Inhaled | 234 | 72.0 (7.0) |
✓ | |||||||||
| 83 | TIVA | 73.0 (8.0) |
● | ● | |||||||||
| 597 | Inhaled | 123 | 71.0 [65-88] |
28 {26-30} |
✓ | ||||||||
| 598 | TIVA | 72.0 [65-88] |
28 {26-30} |
● | |||||||||
| 54 | Inhaled | 1234 | 67.5 {64-71} |
29 {27-29} |
✓ | ||||||||
| 53 | TIVA | 69.0 {64-73} |
29 {28-29} |
● | |||||||||
| Thoracic | |||||||||||||
| 72 | Inhaled | 123 | 72.0 {63-72} |
30 {28-30} |
✓ | ||||||||
| 72 | TIVA | 69.0 {63-73} |
30 {29-30} |
● | |||||||||
| 190 | Inhaled | 123 | 72.4 (5.6) |
28.3 (1.7) |
✓ | ||||||||
| 189 | TIVA | 72.8 (5.5) |
28.2 (1.7) |
● | |||||||||
| 52 | Inhaled | 67.6 (2.5) |
28.6 (1.0) |
✓ | |||||||||
| 52 | TIVA | 67.6 (2.5) |
28.8 (1.0) |
● | |||||||||
| 31 | Inhaled | 12 | 65.5 (16.2) |
28.9 (1.5) |
✓ | ||||||||
| 31 | TIVA | 68.3 (13.5) |
28.3 (1.4) |
● | |||||||||
| Ophthalmologic | |||||||||||||
| 62 | Inhaled | 123 | 76.0 (6.0) |
✓ | ○ | ||||||||
| 62 | TIVA | 77.0 (6.0) |
● | ● | |||||||||
| 32 | Inhaled | 123 | 77.0 (7.0) |
✓e | |||||||||
| 32 | Inhaled | 76.0 (6.0) |
✓ | ○ | |||||||||
| 32 | TIVA | 74.0 (7.0) |
● | ||||||||||
| Otolaryngological | |||||||||||||
| 30 | Inhaled | 68.0 (3.0) |
27.8 (1.8) |
✓ | |||||||||
| 30 | TIVA | 68.0 (2.0) |
28.6 (1.1) |
● | |||||||||
| 31 | Inhaled | 12 | 66.7 (7.2) |
27 {26-29} |
✓ | ○ | |||||||
| 32 | TIVA | 63.9 (5.3) |
28 {26-28} |
● | ● | ||||||||
| Orthopedic | |||||||||||||
| 45 | Inhaled | 123 | 69.8 (4.3) |
✓ | ○ | ○ | |||||||
| 45 | TIVA | 70.6 (5.0) |
● | ||||||||||
| 103 | Inhaled | 123 | 71.5 (6.8) |
26.3 (2.0) |
✓ | ||||||||
| 106 | TIVA | 70.9 (6.7) |
26.2 (1.8) |
● | |||||||||
| Cardiac | |||||||||||||
| 21 | Inhaled | 69.0 (7.0) |
27.5 (2.0) |
✓ | |||||||||
| 23 | TIVA | 67.0 (9.0) |
27.0 (3.0) |
● | |||||||||
| 40 | Inhaled | 69.0 (3.0) |
28.7 (1.3) |
✓ | ○ | ||||||||
| 40 | TIVA | 66.0 (4.0) |
28.8 (1.3) |
● | ● | ||||||||
| 48 | Inhaled | 34 | 64.5 (9.4) |
✓ | |||||||||
| 47 | TIVA | 66.0 (7.3) |
● | ● | |||||||||
| Urologic | |||||||||||||
| 50 | Inhaled | 123 | 69.8 (3.9) |
✓ | ○ | ||||||||
| 50 | TIVA | 69.2 (4.8) |
● | ● | |||||||||
| Head & Neck | |||||||||||||
| 29 | Inhaled | 3 | 69.2 (1.7) |
✓ | ○ | ○ | |||||||
| 30 | TIVA | 72.1 (1.5) |
● | ● | |||||||||
| Vascular | |||||||||||||
| 97 | Inhaled | 234 | 69.0 (9.0) |
✓ | ○ | ||||||||
| 96 | TIVA | 67.0 (9.0) |
● | ● | |||||||||
| Spine | |||||||||||||
| 70 | Inhaled | 23 | 70.1 (3.5) |
✓ | |||||||||
| 70 | TIVA | 69.5 (3.3) |
● | ||||||||||
| TIVA: total intravenous anesthesia; NR: not reported; ASA PS: ASA Physical Status; MMSE: Mini-Mental State Exam; Des: desflurane; Iso: isoflurane; Sev: sevoflurane; Fen: fentanyl; Pro: propofol; Rem: remifentanil; Suf: sufentanil; Oth: other. | |||||||||||||
| a Not reported if none specified. | |||||||||||||
| b Mean Med (SD)[Range]{IQR}. | |||||||||||||
| c Thimylal. | |||||||||||||
| d Induction with sevoflurane; either inhalant used. | |||||||||||||
| e Induction with sevoflurane. | |||||||||||||
Nonrandomized
| Study | N | Comparator | ASA | Agea | MMSEa | Inhaled | TIVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS | Des | Iso | Sev | Fen | Pro | Rem | Suf | |||||
| Cardiac - Retrospective Cohort | ||||||||||||
| 5,325 | Inhaled | 64.9 |
✓ | |||||||||
| 5,210 | TIVA | 64.7 |
● | ● | ||||||||
| 58 | Inhaled | 63.0 (10.0) |
✓ | |||||||||
| 48 | TIVA | 66.0 (11.0) |
● | |||||||||
| 32 | Inhaled | 78.3 (9.0) |
✓b | ✓ | b | ○ | ||||||
| 84 | TIVA | 79.6 (8.7) |
● | ● | ||||||||
| Various - Retrospective Cohort | ||||||||||||
| 386 | Inhaled | 1234 | 65.5 (14.8) |
c | c | c | ||||||
| 100 | TIVA | 65.6 (14.9) |
● | ● | ||||||||
| 1,202 | Inhaled | 34 | 62.7 (13.7) |
✓b | ✓b | |||||||
| 1,374 | TIVA | 65.6 (12.8) |
● | ● | ||||||||
| 427,695 | Inhaled | 76.3 |
✓e | ✓e | ✓e | |||||||
| 142,565 | TIVA | 76.6 |
● | |||||||||
| GI/Abdominal - Retrospective Cohort | ||||||||||||
| 84 | Inhaled | 123 | 67.9 (13.6) |
✓ | ||||||||
| 84 | TIVA | 68.1 (14.0) |
● | |||||||||
| 390 | Inhaled | 1234 | 61.7 (13.4) |
✓b | ✓b | |||||||
| 390 | TIVA | 61.5 (12.4) |
● | ● | ||||||||
| 8,722 | Inhaled | 1234 | 71.0 {63-78} |
✓ | ||||||||
| 8,722 | TIVA | 71.0 {64-77} |
● | |||||||||
| General|Spine|Thoracic|Urol - Prospective Cohort | ||||||||||||
| 36 | Inhaled | 1234 | 76.3 (5.8) |
27 {26-30} |
✓ | |||||||
| 40 | TIVA | 73.5 (5.0) |
29 {28-30} |
● | ||||||||
| General|Thoracic|Urol - Prospective Cohort | ||||||||||||
| 34 | Inhaled | 73.8 |
✓ | |||||||||
| 43 | TIVA | 73.8 |
● | |||||||||
| Ortho - Prospective Cohort | ||||||||||||
| 121 | Inhaled | 69.9 (6.3) |
28.1 (1.2) |
✓ | ||||||||
| 171 | TIVA | 70.1 (6.7) |
28.2 (1.3) |
● | ||||||||
| Ortho - Retrospective Cohort | ||||||||||||
| 5,140 | Inhaled | 74.4 (7.4) |
✓ | |||||||||
| 5,140 | TIVA | 74.5 (7.2) |
● | |||||||||
| 26 | Inhaled | 4 | 85.6 (7.8) |
✓ | ✓ | |||||||
| 20 | TIVA | 83.8 (9.1) |
● | |||||||||
| Thoracic - Retrospective Cohort | ||||||||||||
| 1,477 | Inhaled | 123 | 65.0 (10.4) |
✓ | ○ | ○ | ||||||
| 1,395 | TIVA | 65.0 (10.0) |
● | ● | ||||||||
| Spine - Retrospective Cohort | ||||||||||||
| 140 | Inhaled | 123 | 72.9 (4.7) |
✓ | ||||||||
| 141 | TIVA | 72.4 (4.5) |
● | |||||||||
| Thoracic - Nonrandomized Trial | ||||||||||||
| 116 | Inhaled | 12 | 63.4 (17.1) |
25.9 (1.2) |
✓ | |||||||
| 149 | TIVA | 67.2 (15.1) |
26.1 (1.4) |
● | ||||||||
| TIVA: total intravenous anesthesia; NR: not reported; ASA PS: ASA Physical Status; MMSE: Mini-Mental State Exam; Des: desflurane; Iso: isoflurane; Sev: sevoflurane; Fen: fentanyl; Pro: propofol; Rem: remifentanil; Suf: sufentanil. | ||||||||||||
| a Mean Med (SD)[Range]{IQR}. | ||||||||||||
| b Either of the two inhalent agents used. | ||||||||||||
| c Agent used not specified. | ||||||||||||
| d End-stage renal disease patients. | ||||||||||||
| e Any agent used. | ||||||||||||
Delirium Incidence
| Study | N | Arm | Scale | Day(s)a | Incidence Proportion | RR OR (95% CI) | |
|---|---|---|---|---|---|---|---|
| N (%) | 0 – 100% | ||||||
| GI/Abd – Randomized Clinical Trial | |||||||
| 25 | Inhaled | DRS | 3 | 0 (0) | |||
| 25 | TIVA | 4 (16.0) | |||||
| 15 | Inhaled | DRS | 3 | 2 (13.3) | — | ||
| 15 | TIVA | 3 (20.0) | 1.50 (0.29-7.73) | ||||
| 30 | Inhaled | CAM | Stay | 8 (26.7) | — | ||
| 29 | TIVA | 2 (6.9) | 0.26 (0.06-1.12) | ||||
| Ortho – Randomized Clinical Trial | |||||||
| 45 | Inhaled | CAM | 2 | 0 (0) | |||
| 45 | TIVA | 1 (2.2) | |||||
| 103 | Inhaled | CAM | 3 | 24 (23.3) | — | ||
| 106 | TIVA | 35 (33.0) | 1.42 (0.91-2.21) | ||||
| Various – Randomized Clinical Trial | |||||||
| 81 | Inhaled | NS | Stay | 36 (44.4) | — | ||
| 83 | TIVA | 35 (42.2) | 0.95 (0.67-1.35) | ||||
| 597 | Inhaled | CAM | 7 | 74 (12.4) | — | ||
| 598 | TIVA | 50 (8.4) | 0.67 (0.48-0.95) | ||||
| 99 | Inhaled | CAM | 3 | 14 (14.1) | — | ||
| 100 | TIVA | 13 (13.0) | 0.92 (0.46-1.85) | ||||
| Cardiac – Retrospective Cohort | |||||||
| 32 | Inhaled | CAM | 2 | 11 (34.4) | — | ||
| 84 | TIVA | 12 (14.3) | 0.22 (0.06-0.79)b | ||||
| Various – Prospective Cohort | |||||||
| 36 | Inhaled | CAM | 3 | 8 (22.2) | — | ||
| 40 | TIVA | 6 (15.0) | 0.62 (0.19-1.99)c | ||||
| Various – Retrospective Cohort | |||||||
| 427,695 | Inhaled | ICD-10d | Stay | 32,912 (7.7) | — | ||
| 142,565 | TIVA | 10,269 (7.2) | 0.93 (0.91-0.95)e | ||||
| Ortho – Retrospective Cohort | |||||||
| 26 | Inhaled | NS | Stay | 2 (7.7) | — | ||
| 20 | TIVA | 3 (15.0) | 2.12 (0.32-14.07)c | ||||
| Spine – Retrospective Cohort | |||||||
| 140 | Inhaled | KNDSS | Stay | 22 (15.7) | — | ||
| 141 | TIVA | 7 (5.0) | 4.12 (1.55-10.95) | ||||
| RR: risk ratio; OR: odds ratio; DRS: Delirium Rating Scale; CAM: Confusion Assessment Method; KNDSS: Korean Nursing Delirium Screening Scale; NS: not specified. | |||||||
| a Day(s) over which incidence proportion assessed. Stay indicates duration of hospitalization. | |||||||
| b Adjusted from multivariable model. | |||||||
| c Calculated crude odds ratio. | |||||||
| d Or new antipsychotic prescription. | |||||||
| e Propensity score matched. | |||||||
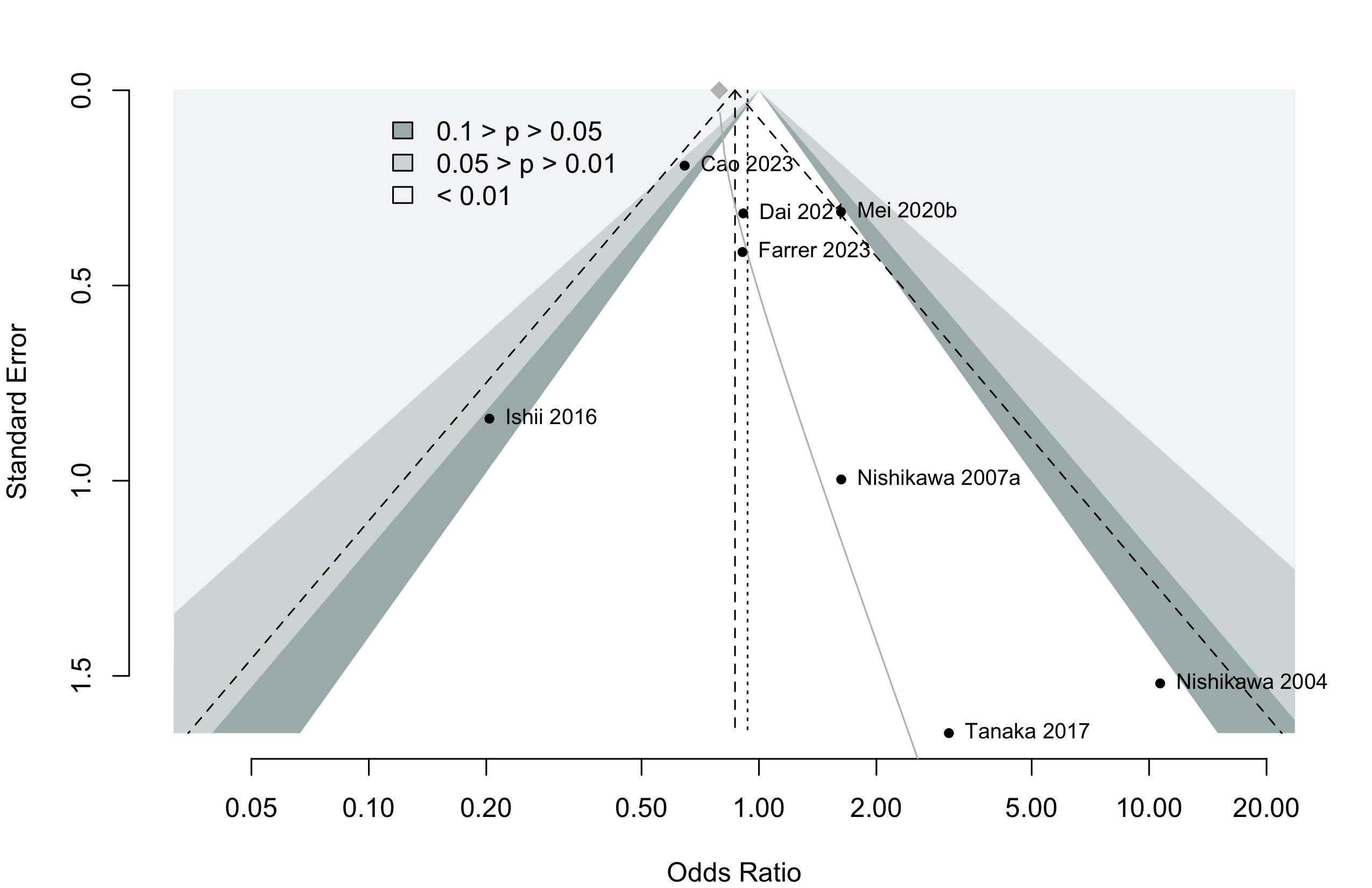
Pooled
Randomized
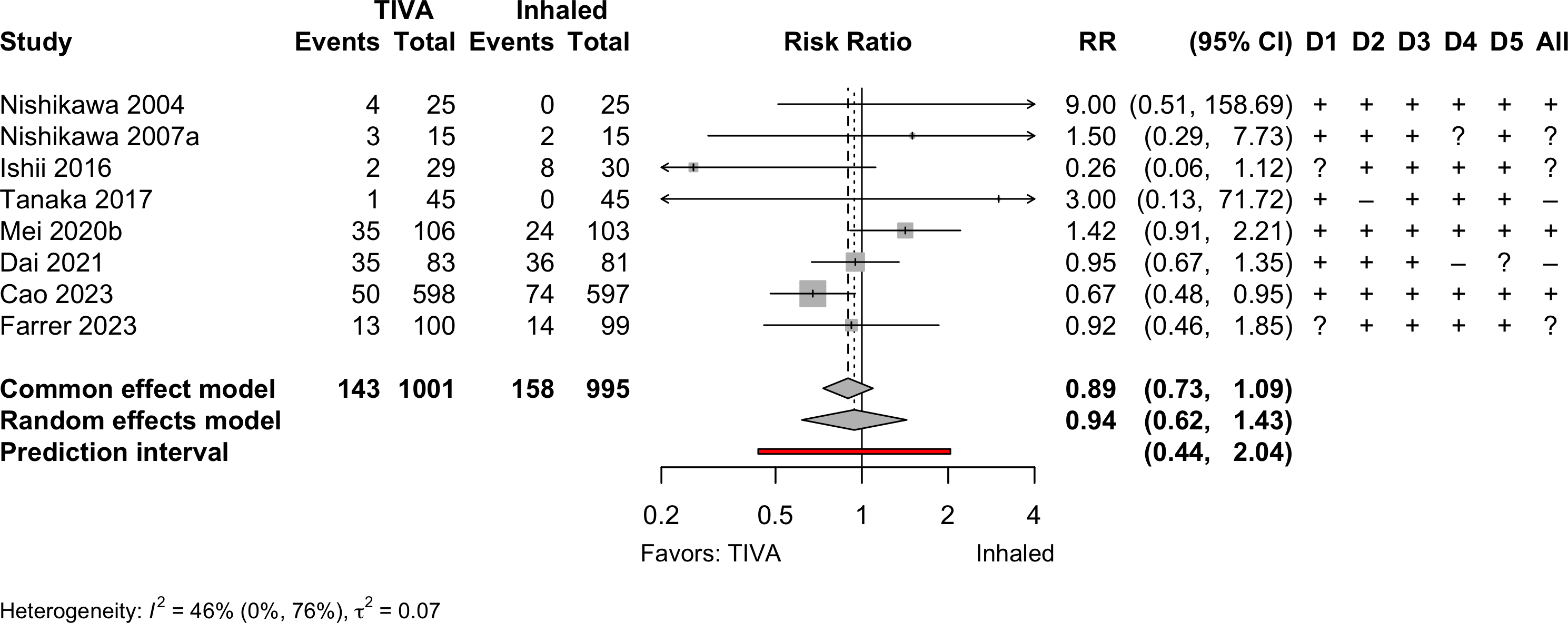
Risk of bias ratings: low +, some concerns ?, high – .
Continuity correction of 0.5 added to studies with no events in one arm.
Nonrandomized
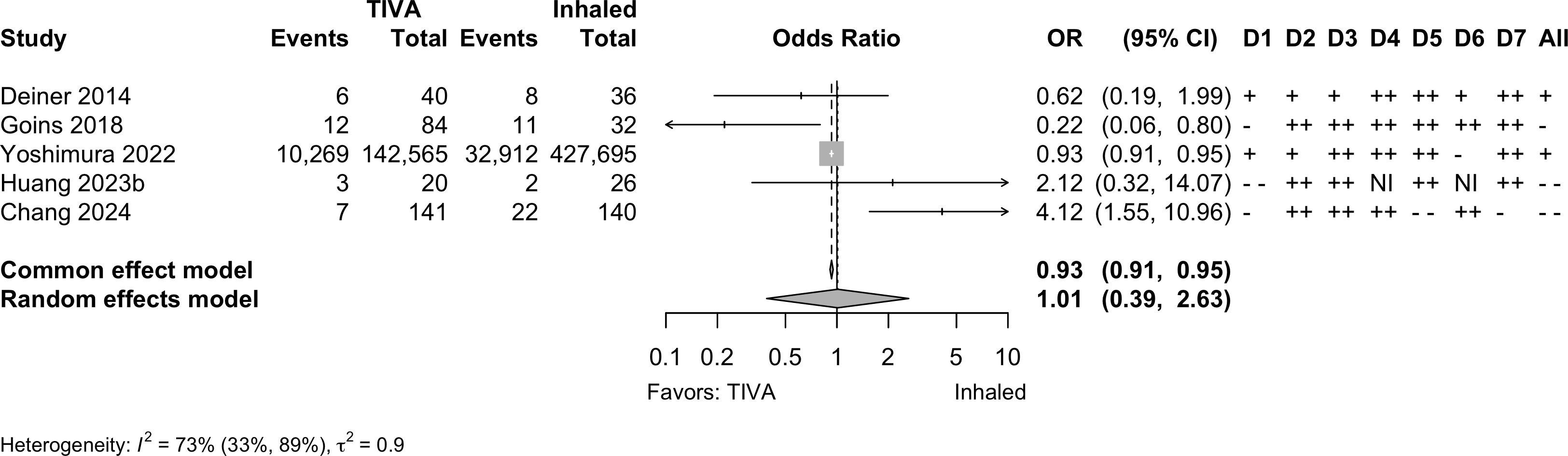
Note: adjusted odds ratios pooled from Yoshimura 2022 (propensity matching); Goins 2018 and Chang 2022 (multivariable adjustment).
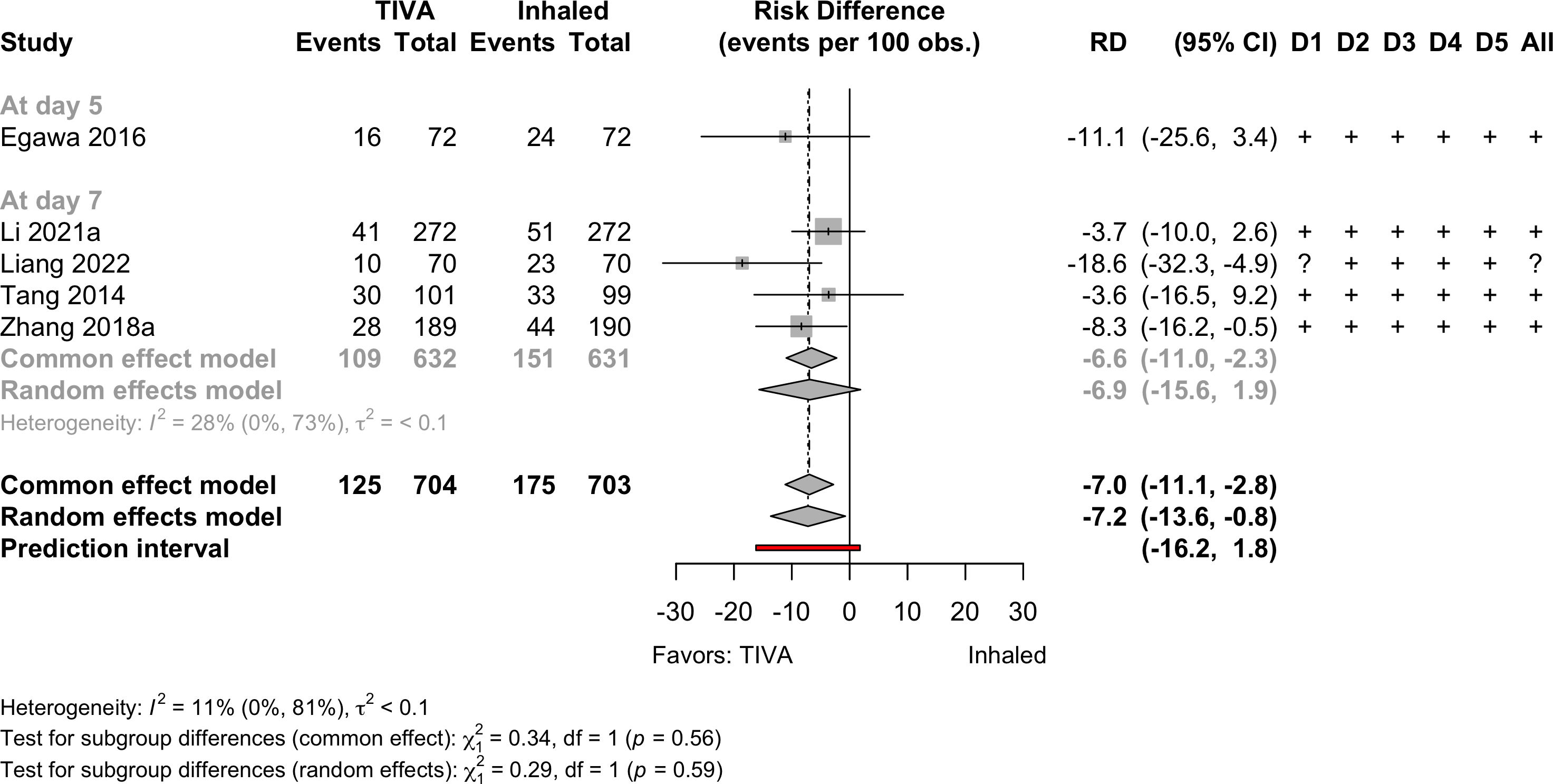
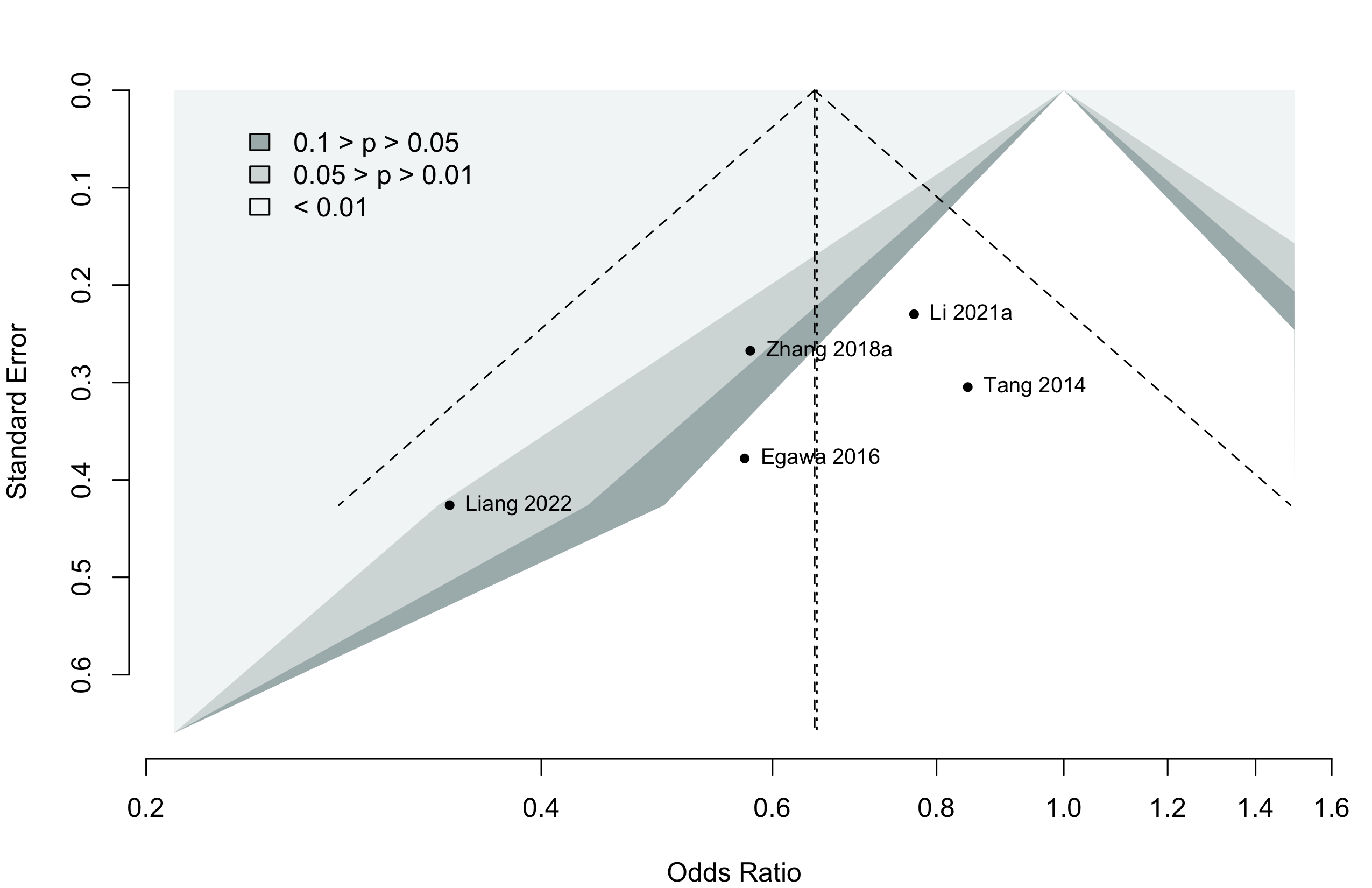
Neurocognitive Disorder
<30 days
| Study | N | Comparator | Preop | Instrument | Dayc | Neurocognitive Disorder <30 days | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MMSEa | MMSE | MoCA | Multipleb | NS | N (%) | 0 — 100% | RR (95% CI) | ||||
| Randomized Clinical Trial — Gastrointestinal/Abdominal | |||||||||||
| 99 | Inhaled | 25.0 (1.7) |
✓d |
7 | 33 (33.3) | — | |||||
| 101 | TIVA | 24.6 (1.7) |
30 (29.7) | 0.89 (0.59-1.34) | |||||||
| 50 | Inhaled | 28.9 (1.4) |
✓e,f |
3 | 10 (20.0) | — | |||||
| 50 | Inhaled | 29.4 (1.9) |
15 (30.0) | 1.50 (0.75-3.01) | |||||||
| 50 | TIVA | 29.2 (1.5) |
2 (4.0) | 0.20 (0.05-0.87) | |||||||
| 272 | Inhaled | 29 {28-30} |
✓d |
7 | 51 (18.8) | — | |||||
| 272 | TIVA | 29 {28-30} |
41 (15.1) | 0.80 (0.55-1.17) | |||||||
| Randomized Clinical Trial — Thoracic | |||||||||||
| 72 | Inhaled | 30 {28-30} |
✓f |
5 | 24 (33.3) | — | |||||
| 72 | TIVA | 30 {29-30} |
16 (22.2) | 0.67 (0.39-1.15) | |||||||
| 190 | Inhaled | 28.3 (1.7) |
✓d |
7 | 44 (23.2) | — | |||||
| 189 | TIVA | 28.2 (1.7) |
28 (14.8) | 0.64 (0.42-0.98) | |||||||
| Randomized Clinical Trial — Spine | |||||||||||
| 70 | Inhaled | ✓g |
7 | 23 (32.9) | — | ||||||
| 70 | TIVA | 10 (14.3) | 0.43 (0.22-0.84) | ||||||||
| Prospective Cohort — Orthopedic | |||||||||||
| 119 | Inhaled | 28.1 (1.2) |
✓d |
7 | 24 (20.2) | — | |||||
| 160 | TIVA | 28.2 (1.3) |
24 (15.0) | 0.74 (0.44-1.24) | |||||||
| MMSE: Mini-Mental State Exam; MoCA: MoCA: Montreal Cognitive Assessment; NS: not specfied; RR: risk ratio; CI: confidence interval. | |||||||||||
| a Mean Med (SD)[Range]{IQR}. | |||||||||||
| b Failed 2 or more tests. | |||||||||||
| c Day of assessment. | |||||||||||
| d Z ≥1.96. | |||||||||||
| e Digit Span Test; Digit Symbol Test; Grooved Pegboard Test; Mini-Mental State Examination; Rey Auditory Verbal Learning; Trail Marking Test A. | |||||||||||
| f Difference from baseline >20%. | |||||||||||
| g Difference from baseline ≥1 SD. | |||||||||||
Pooled

Risk of bias ratings: low +, some concerns ?, high – .
Four trials conducted in China and one each in Norway (Lindholm 2013) and Japan (Egawa 2016).
Including Geng 2017 assessments at day 3 — RR 0.66 (95% CI, 0.54–0.81; prediction interval, 0.54–0.91)
Neurocognitive Disorder
30 days to 1 year
| Study | N | Comparator | Preop | Instrument | Dayb | Neurocognitive Disorder ≥30 days | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MMSEa | MMSE | MoCA | Other | NS | N (%) | 0 — 100% | RR (95% CI) | ||||
| Randomized Clinical Trial | |||||||||||
| 97 | Inhaled | ✓ | 30 | 6 (6.2) | — | ||||||
| 96 | TIVA | 4 (4.2) | 0.67 (0.20-2.31) | ||||||||
| Prospective Cohort | |||||||||||
| 34 | Inhaled | ✓c | 90 | 9 (26.5) | — | ||||||
| 43 | TIVA | 12 (27.9) | 1.05 (0.50-2.21) | ||||||||
| 115 | Inhaled | 28.1 (1.2) |
✓d | 90 | 10 (8.7) | — | |||||
| 161 | TIVA | 28.2 (1.3) |
17 (10.6) | 1.21 (0.58-2.55) | |||||||
| Retrospective Cohort | |||||||||||
| 58 | Inhaled | ✓e,f | 180 | 13 (22.4) | — | ||||||
| 48 | TIVA | 11 (22.9) | 1.02 (0.50-2.07) | ||||||||
| Mini-Mental State Exam; MoCA: Montreal Cognitive Assessment; NS: not stated; RR: risk ratio. | |||||||||||
| Pooled RR 1.10 (95% CI, 0.72–1.68) | |||||||||||
| a Mean Med (SD)[Range]{IQR}. | |||||||||||
| b Day of assessment. | |||||||||||
| c Uniform Data Set of the Alzheimer’s Disease Centers. | |||||||||||
| d Z ≥1.96. | |||||||||||
| e Digit Span Test; Grooved Pegboard Test; Mini-Mental State Examination; Rey Auditory Verbal Learning; Trail Marking Test A; Trail Making Test B. | |||||||||||
| f Failed 2 or more tests. | |||||||||||
Pooled
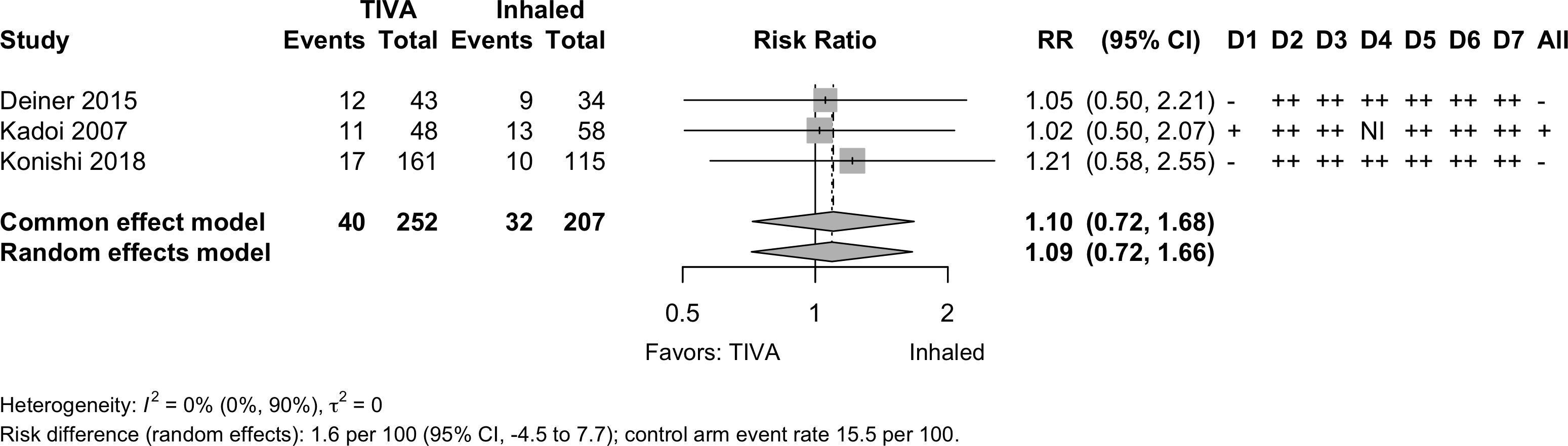
Physical Function
No studies
Complications
| Study | N | Arm | Agea | Surgery | N (%) | 0 – 100% | RD OR (95% CI)b |
|---|---|---|---|---|---|---|---|
| Myocardial Infarction – Randomized Clinical Trial | |||||||
| 44 | Inhaled | 65.0 (11.8) |
Various | 0 (0) | — | ||
| 44 | TIVA | 64.0 (12.2) |
0 (0) | 0.00% (-4.33, 4.33) | |||
| 97 | Inhaled | 69.0 (9.0) |
Vascular | 5 (5.2) | — | ||
| 96 | TIVA | 67.0 (9.0) |
3 (3.1) | -2.03% (-7.64, 3.58) | |||
| Myocardial Infarction – Retrospective Cohort | |||||||
| 5,325 | Inhaled | 64.9 |
Cardiac | 2,076 (39.0) | — | ||
| 5,210 | TIVA | 64.7 |
1,891 (36.3) | -2.69% (-4.54, -0.84) | |||
| 1,202 | Inhaled | 62.7 (13.7) |
Various | 15 (1.2) | — | ||
| 1,374 | TIVA | 65.6 (12.8) |
6 (0.4) | -0.81% (-1.53, -0.09) | |||
| 8,722 | Inhaled | 71.0 {63-78} |
GI/Abd | 82 (0.9) | — | ||
| 8,722 | TIVA | 71.0 {64-77} |
88 (1.0) | 0.93 (0.69—1.26) | |||
| 427,695 | Inhaled | 76.3 |
Various | 6,717 (1.6) | — | ||
| 142,565 | TIVA | 76.6 |
2,039 (1.4) | 0.91 (0.86—0.96) | |||
| 26 | Inhaled | 85.6 (7.8) |
Ortho | 3 (11.5) | — | ||
| 20 | TIVA | 83.8 (9.1) |
0 (0) | -11.54% (-25.79, 2.71) | |||
| Cardiac Arrest – Randomized Clinical Trial | |||||||
| 597 | Inhaled | 71.0 [65-88] |
Various | 1 (0.2) | — | ||
| 598 | TIVA | 72.0 [65-88] |
3 (0.5) | 0.33% (-0.32, 0.99) | |||
| Cardiac Arrest – Retrospective Cohort | |||||||
| 1,202 | Inhaled | 62.7 (13.7) |
Various | 0 (0) | — | ||
| 1,374 | TIVA | 65.6 (12.8) |
2 (0.1) | 0.15% (-0.11, 0.40) | |||
| Bradycardia – Randomized Clinical Trial | |||||||
| 32 | Inhaled | 77.0 (7.0) |
Ophtho | 1 (3.1) | — | ||
| 32 | Inhaled | 76.0 (6.0) |
4 (12.5) | — | |||
| 32 | TIVA | 74.0 (7.0) |
8 (25.0) | 17.19% (0.81, 33.57)d | |||
| 15 | Inhaled | 70.9 (6.5) |
GI/Abd | 2 (13.3) | — | ||
| 15 | TIVA | 71.2 (5.3) |
0 (0) | -13.33% (-33.06, 6.40) | |||
| 190 | Inhaled | 72.4 (5.6) |
Thoracic | 8 (4.2) | — | ||
| 189 | TIVA | 72.8 (5.5) |
6 (3.2) | -1.04% (-4.83, 2.76) | |||
| 31 | Inhaled | 65.5 (16.2) |
Thoracic | 0 (0) | — | ||
| 31 | TIVA | 68.3 (13.5) |
1 (3.2) | 3.23% (-5.27, 11.72) | |||
| Bradycardia – Nonrandomized Trial | |||||||
| 116 | Inhaled | 63.4 (17.1) |
Thoracic | 1 (0.9) | — | ||
| 149 | TIVA | 67.2 (15.1) |
5 (3.4) | 2.49% (-0.85, 5.84) | |||
| Hypotension – Randomized Clinical Trial | |||||||
| 31 | Inhaled | 65.5 (16.2) |
Thoracic | 3 (9.7) | — | ||
| 31 | TIVA | 68.3 (13.5) |
1 (3.2) | -6.45% (-18.58, 5.67) | |||
| 597 | Inhaled | 71.0 [65-88] |
Various | 101 (16.9) | — | ||
| 598 | TIVA | 72.0 [65-88] |
97 (16.2) | -0.70% (-4.91, 3.52) | |||
| Hypotension – Nonrandomized Trial | |||||||
| 116 | Inhaled | 63.4 (17.1) |
Thoracic | 11 (9.5) | — | ||
| 149 | TIVA | 67.2 (15.1) |
5 (3.4) | -6.13% (-12.19, -0.06) | |||
| Hypotension – Retrospective Cohort | |||||||
| 140 | Inhaled | 72.9 (4.7) |
Spine | 122 (87.1) | — | ||
| 141 | TIVA | 72.4 (4.5) |
123 (87.2) | 0.09% (-7.72, 7.91) | |||
| Other Cardiac – Randomized Clinical Trial | |||||||
| 44 | Inhaled | 65.0 (11.8) |
Various | 1 (2.3) | — | ||
| 44 | TIVA | 64.0 (12.2) |
1 (2.3) | 0.00% (-6.23, 6.23) | |||
| 81 | Inhaled | 72.0 (7.0) |
Various | 2 (2.5) | — | ||
| 83 | TIVA | 73.0 (8.0) |
2 (2.4) | -0.06% (-4.78, 4.66) | |||
| 597 | Inhaled | 71.0 [65-88] |
Various | 9 (1.5) | — | ||
| 598 | TIVA | 72.0 [65-88] |
13 (2.2) | 0.67% (-0.86, 2.19) | |||
| Stroke – Randomized Clinical Trial | |||||||
| 597 | Inhaled | 71.0 [65-88] |
Various | 3 (0.5) | — | ||
| 598 | TIVA | 72.0 [65-88] |
3 (0.5) | -0.00% (-0.80, 0.80) | |||
| Stroke – Retrospective Cohort | |||||||
| 8,722 | Inhaled | 71.0 {63-78} |
GI/Abd | 39 (0.4) | — | ||
| 8,722 | TIVA | 71.0 {64-77} |
34 (0.4) | 1.15 (0.72—1.83) | |||
| Acute Kidney Injury – Randomized Clinical Trial | |||||||
| 597 | Inhaled | 71.0 [65-88] |
Various | 36 (6.0) | — | ||
| 598 | TIVA | 72.0 [65-88] |
38 (6.4) | 0.32% (-2.41, 3.06) | |||
| Acute Kidney Injury – Retrospective Cohort | |||||||
| 1,477 | Inhaled | 65.0 (10.4) |
Thoracic | 71 (4.8) | — | ||
| 1,395 | TIVA | 65.0 (10.0) |
69 (4.9) | 0.96 (0.53—1.71) | |||
| 386 | Inhaled | 65.5 (14.8) |
Various | 47 (12.2) | — | ||
| 100 | TIVA | 65.6 (14.9) |
7 (7.0) | 0.44 (0.18—0.95) | |||
| 427,695 | Inhaled | 76.3 |
Various | 2,123 (0.5) | — | ||
| 142,565 | TIVA | 76.6 |
706 (0.5) | 1.00 (0.91—1.09) | |||
| 26 | Inhaled | 85.6 (7.8) |
Ortho | 4 (15.4) | — | ||
| 20 | TIVA | 83.8 (9.1) |
1 (5.0) | -10.38% (-27.22, 6.45) | |||
| 140 | Inhaled | 72.9 (4.7) |
Spine | 2 (1.4) | — | ||
| 141 | TIVA | 72.4 (4.5) |
2 (1.4) | -0.01% (-2.78, 2.76) | |||
| Pneumonia – Randomized Clinical Trial | |||||||
| 97 | Inhaled | 69.0 (9.0) |
Vascular | 13 (13.4) | — | ||
| 96 | TIVA | 67.0 (9.0) |
9 (9.4) | -4.03% (-12.97, 4.91) | |||
| 153 | Inhaled | 66.0 [19-93] |
GI/Abd | 8 (5.2) | — | ||
| 154 | TIVA | 65.0 [23-89] |
7 (4.5) | -0.68% (-5.51, 4.14) | |||
| 272 | Inhaled | 65.0 {62-69} |
GI/Abd | 1 (0.4) | — | ||
| 272 | TIVA | 64.0 {62-68} |
0 (0) | -0.37% (-1.38, 0.65) | |||
| Pneumonia – Retrospective Cohort | |||||||
| 8,722 | Inhaled | 71.0 {63-78} |
GI/Abd | 303 (3.5) | — | ||
| 8,722 | TIVA | 71.0 {64-77} |
275 (3.2) | 1.11 (0.94—1.31) | |||
| 26 | Inhaled | 85.6 (7.8) |
Ortho | 10 (38.5) | — | ||
| 20 | TIVA | 83.8 (9.1) |
2 (10.0) | -28.46% (-51.32, -5.60) | |||
| Pneumothorax – Randomized Clinical Trial | |||||||
| 597 | Inhaled | 71.0 [65-88] |
Various | 3 (0.5) | — | ||
| 598 | TIVA | 72.0 [65-88] |
6 (1.0) | 0.50% (-0.48, 1.48) | |||
| Pulmonary Embolism – Randomized Clinical Trial | |||||||
| 153 | Inhaled | 66.0 [19-93] |
GI/Abd | 0 (0) | — | ||
| 154 | TIVA | 65.0 [23-89] |
2 (1.3) | 1.30% (-0.88, 3.48) | |||
| 597 | Inhaled | 71.0 [65-88] |
Various | 1 (0.2) | — | ||
| 598 | TIVA | 72.0 [65-88] |
1 (0.2) | -0.00% (-0.46, 0.46) | |||
| Pulmonary Embolism – Retrospective Cohort | |||||||
| 1,202 | Inhaled | 62.7 (13.7) |
Various | 0 (0) | — | ||
| 1,374 | TIVA | 65.6 (12.8) |
2 (0.1) | 0.15% (-0.11, 0.40) | |||
| 8,722 | Inhaled | 71.0 {63-78} |
GI/Abd | 32 (0.4) | — | ||
| 8,722 | TIVA | 71.0 {64-77} |
22 (0.3) | 1.46 (0.85—2.54) | |||
| 427,695 | Inhaled | 76.3 |
Various | 432 (0.1) | — | ||
| 142,565 | TIVA | 76.6 |
185 (0.1) | 1.29 (1.06—1.53) | |||
| Pulmonary Edema – Retrospective Cohort | |||||||
| 1,202 | Inhaled | 62.7 (13.7) |
Various | 3 (0.2) | — | ||
| 1,374 | TIVA | 65.6 (12.8) |
0 (0) | -0.25% (-0.57, 0.07) | |||
| 427,695 | Inhaled | 76.3 |
Various | 0 (0) | — | ||
| 142,565 | TIVA | 76.6 |
0 (0) | — | |||
| Respiratory Failure – Randomized Clinical Trial | |||||||
| 97 | Inhaled | 69.0 (9.0) |
Vascular | 9 (9.3) | — | ||
| 96 | TIVA | 67.0 (9.0) |
6 (6.2) | -3.03% (-10.56, 4.51) | |||
| 597 | Inhaled | 71.0 [65-88] |
Various | 6 (1.0) | — | ||
| 598 | TIVA | 72.0 [65-88] |
8 (1.3) | 0.33% (-0.89, 1.55) | |||
| Respiratory Failure – Retrospective Cohort | |||||||
| 8,722 | Inhaled | 71.0 {63-78} |
GI/Abd | 216 (2.5) | — | ||
| 8,722 | TIVA | 71.0 {64-77} |
236 (2.7) | 0.91 (0.76—1.10) | |||
| 427,695 | Inhaled | 76.3 |
Various | 1,487 (0.3) | — | ||
| 142,565 | TIVA | 76.6 |
420 (0.3) | 0.85 (0.76—0.94) | |||
| RD: risk difference; OR: odds ratio; Ophtho: ophthalmologic; GI: gastointestinal; GI: gastrointestinal; Abd: abdominal. | |||||||
| a Mean Med (SD)[Range]{IQR}. | |||||||
| b Odds ratios for propensity-matched studies (risk differences accounting for matching were not reported). | |||||||
| c Definition not reported. | |||||||
| d Compared with combined inhalation arms (differed only in induction agents). | |||||||
| e <50 bpm. | |||||||
| f <50 bpm or ↓30% and require chronotropic agent. | |||||||
| g Systolic blood pressure <90 mm Hg or a decrease of >30% from baseline. | |||||||
| h Atrial fibrillation. | |||||||
| i Cardiac dysfunction. | |||||||
| j Arrhythmia. | |||||||
Pooled
Note: given the limited number of randomized studies and absence of convincing evidence for any complication, randomized and nonrandomized designs were poole without detriment to any strength of evidence rating. When odds ratios were pooled (to include adjusted results from nonrandomized designs), approximate risk differences were calculated based on the event rate across inhaled anesthetic arms and risk ratio derived from the odds ratio.
Myocardial Infarction

Cardiac Arrest
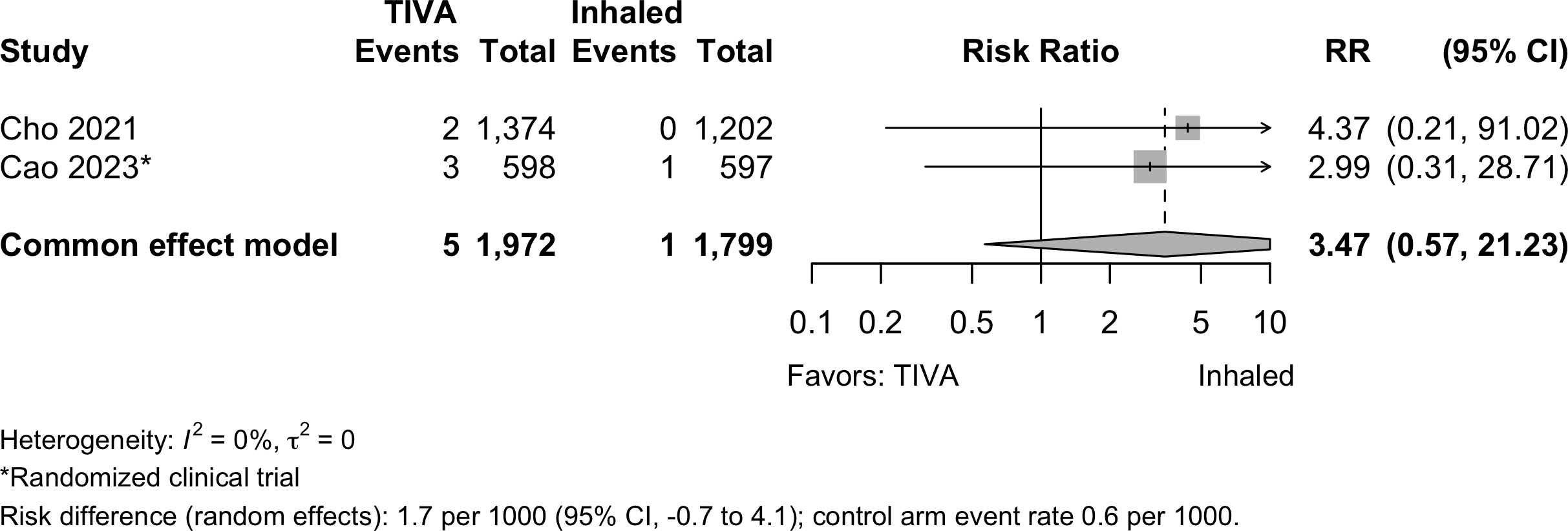
Bradycardia
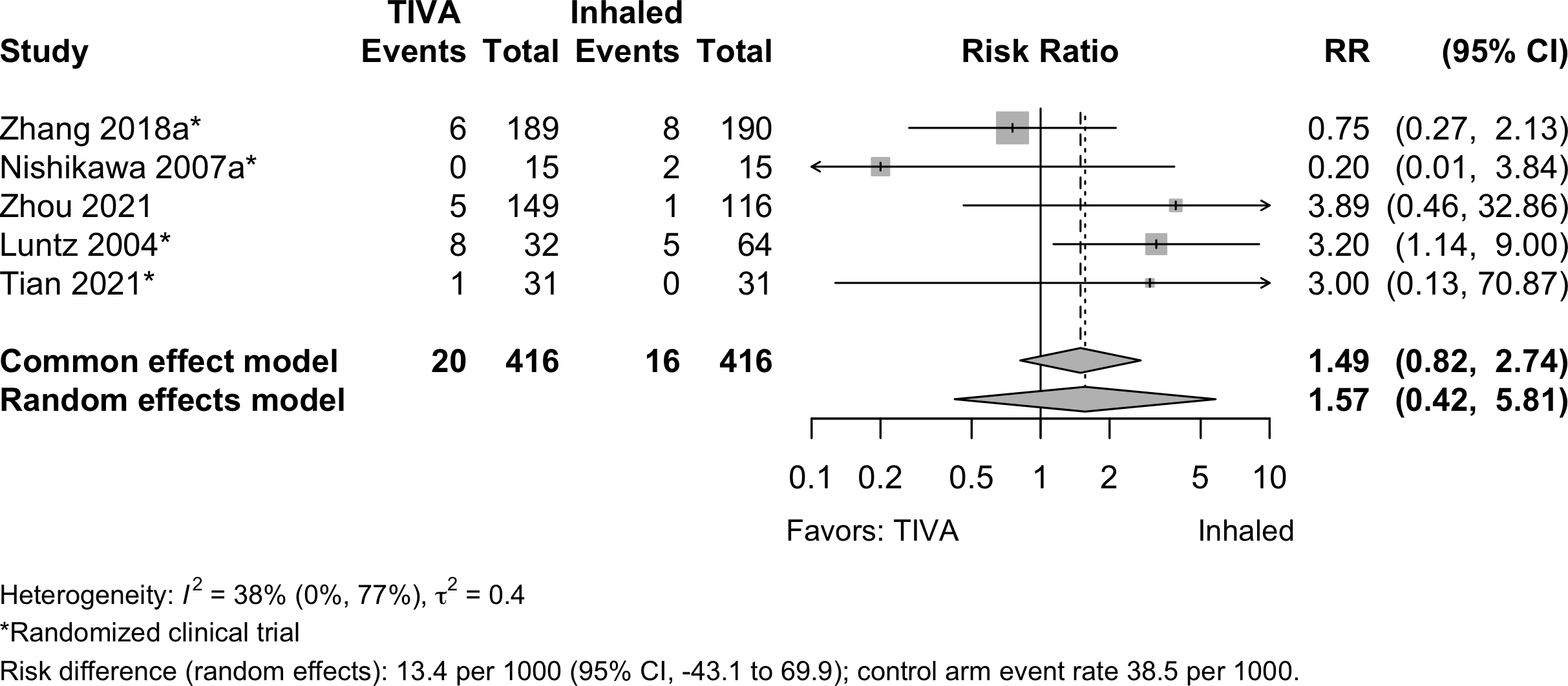
Hypotension
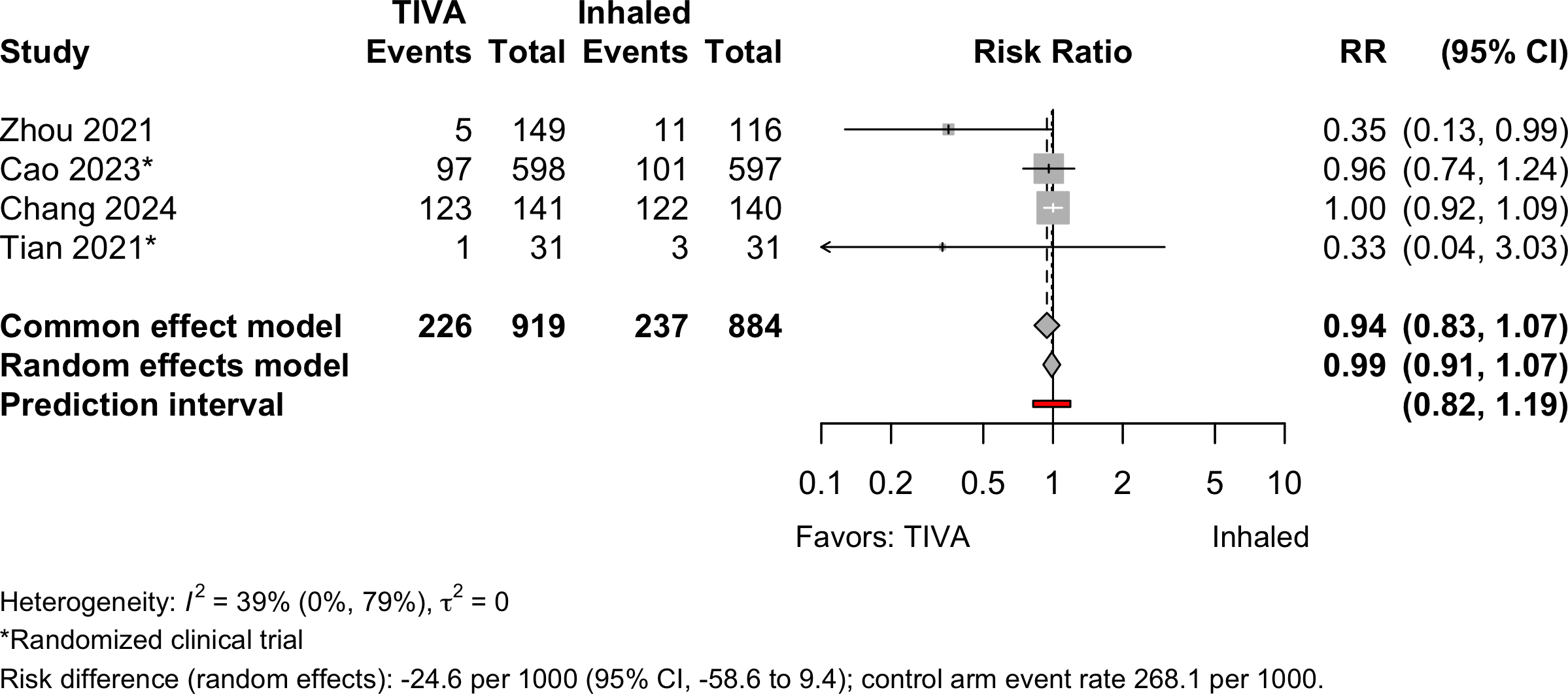
Stroke

Acute Kidney Injury
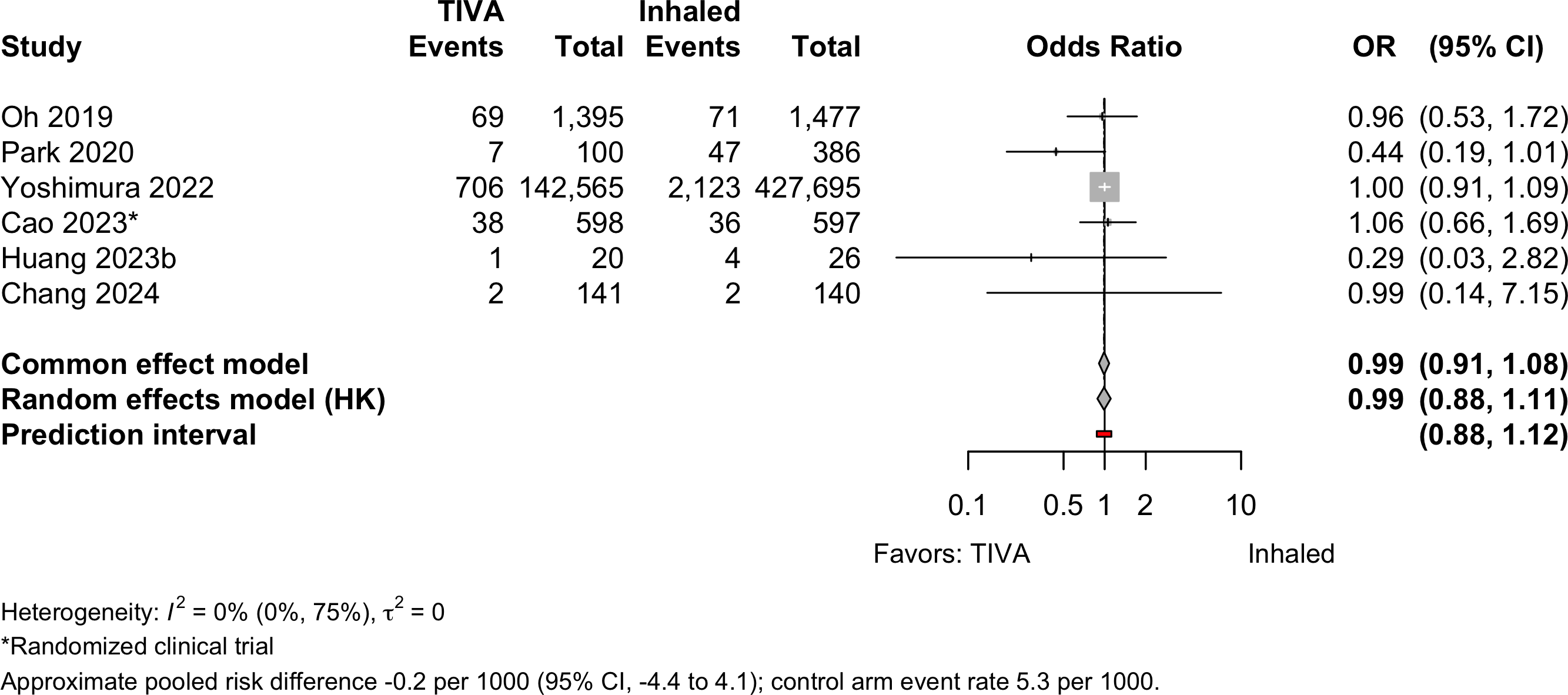
Pneumonia
Pulmonary Embolism
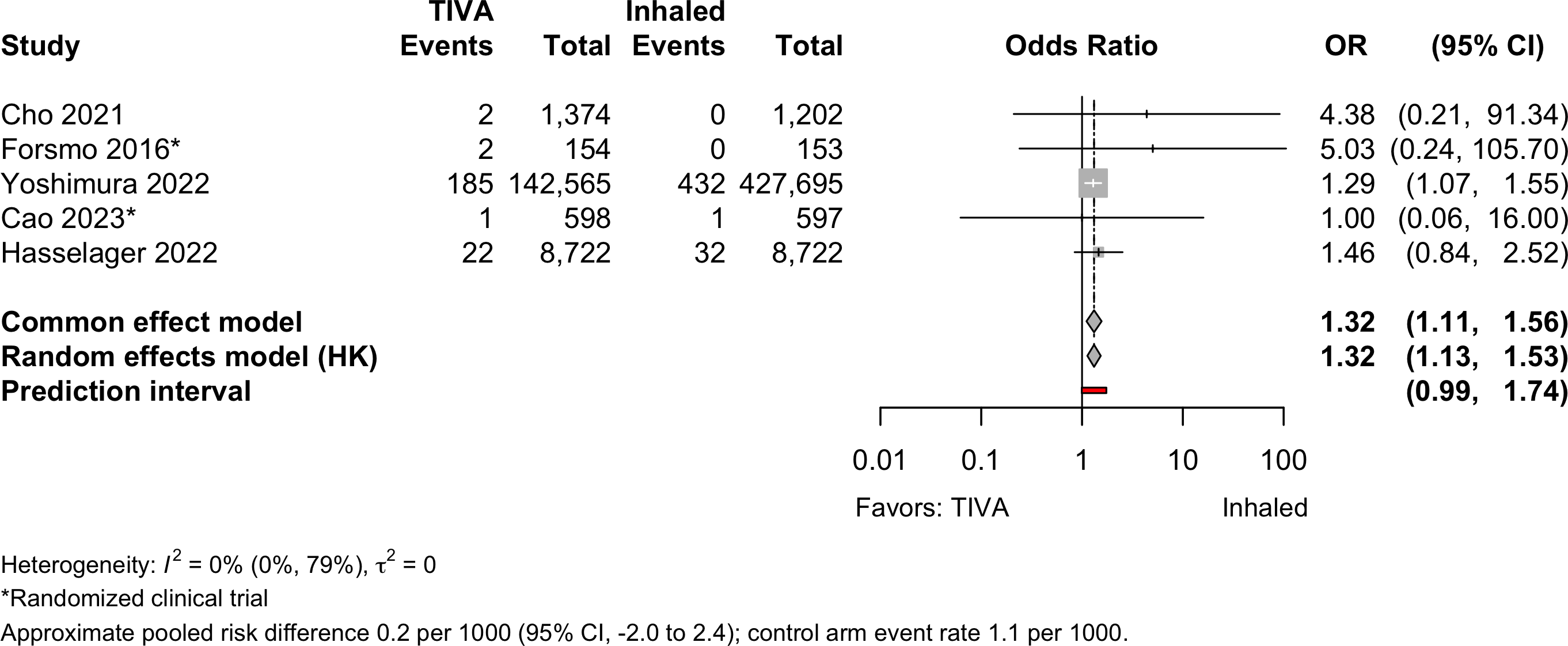
Respiratory Failure
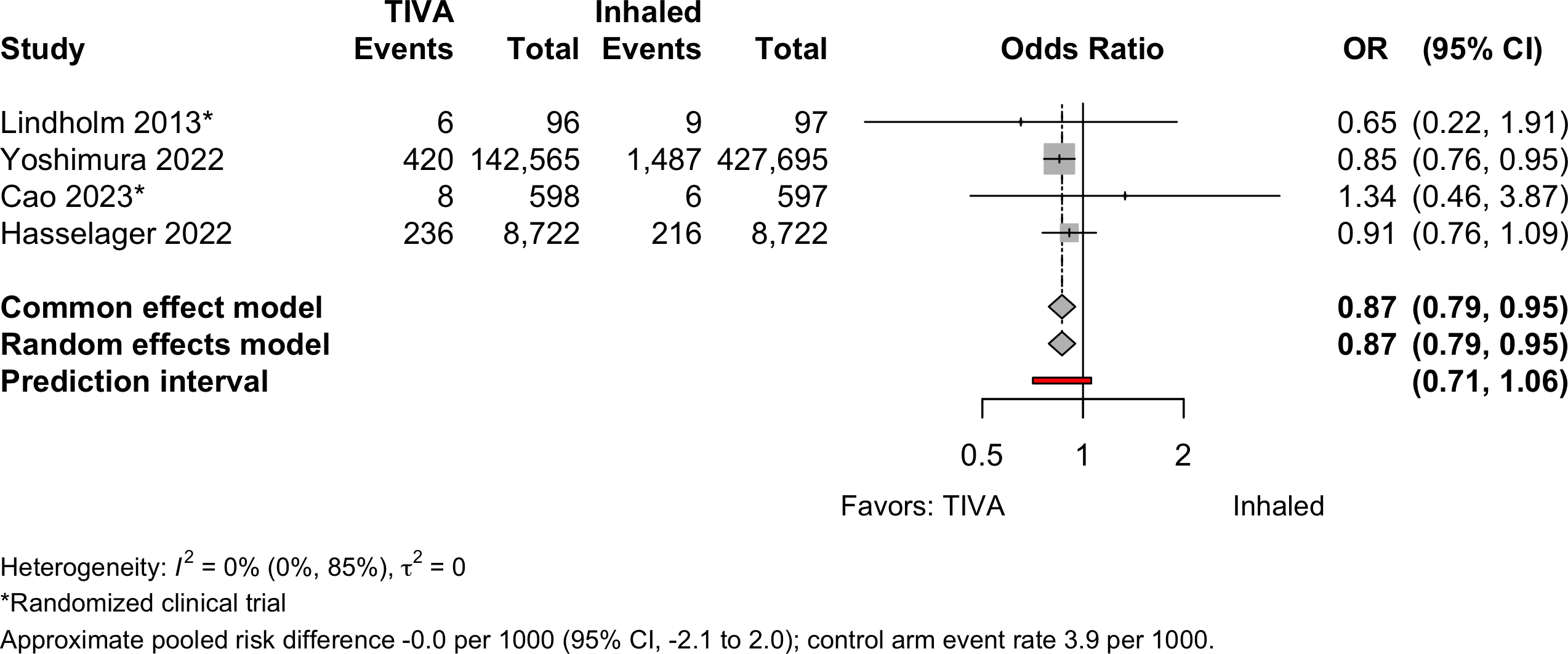
Patient Satisfaction
| Study | N | Anesth | Surgery | ASA | Agea | N (%) | 0 – 100% | RD (95% CI) |
|---|---|---|---|---|---|---|---|---|
| PS | ||||||||
| 62 | Inhaled | Ophthalmologic | 123 | 76.0 (6.0) |
41 (66.1)b | — | ||
| 62 | TIVA | 77.0 (6.0) |
58 (93.5)b | 27.4% (14.1, 40.7) | ||||
| 64 | Inhaledc | Ophthalmologic | 123 | 77.0 (7.0) |
15 (46.9)d | — | ||
| 32 | TIVA | 74.0 (7.0) |
23 (71.9)d | 17.2% (-2.6, 37.0) | ||||
| 15 | Inhaled | GI/Abdominal | 12 | 70.9 (6.5) |
6 (40.0)e | — | ||
| 15 | TIVA | 71.2 (5.3) |
9 (60.0)e | 20.0% (-15.1, 55.1) | ||||
| TIVA: total intravenous anesthesia; ASA PS: ASA Physical Status; RD: risk difference. | ||||||||
| a Mean (SD). | ||||||||
| b Completely satisfied. | ||||||||
| c Inhaled arms combined. | ||||||||
| d Highly satisfied. | ||||||||
| e Very satisfied. | ||||||||
Pooled
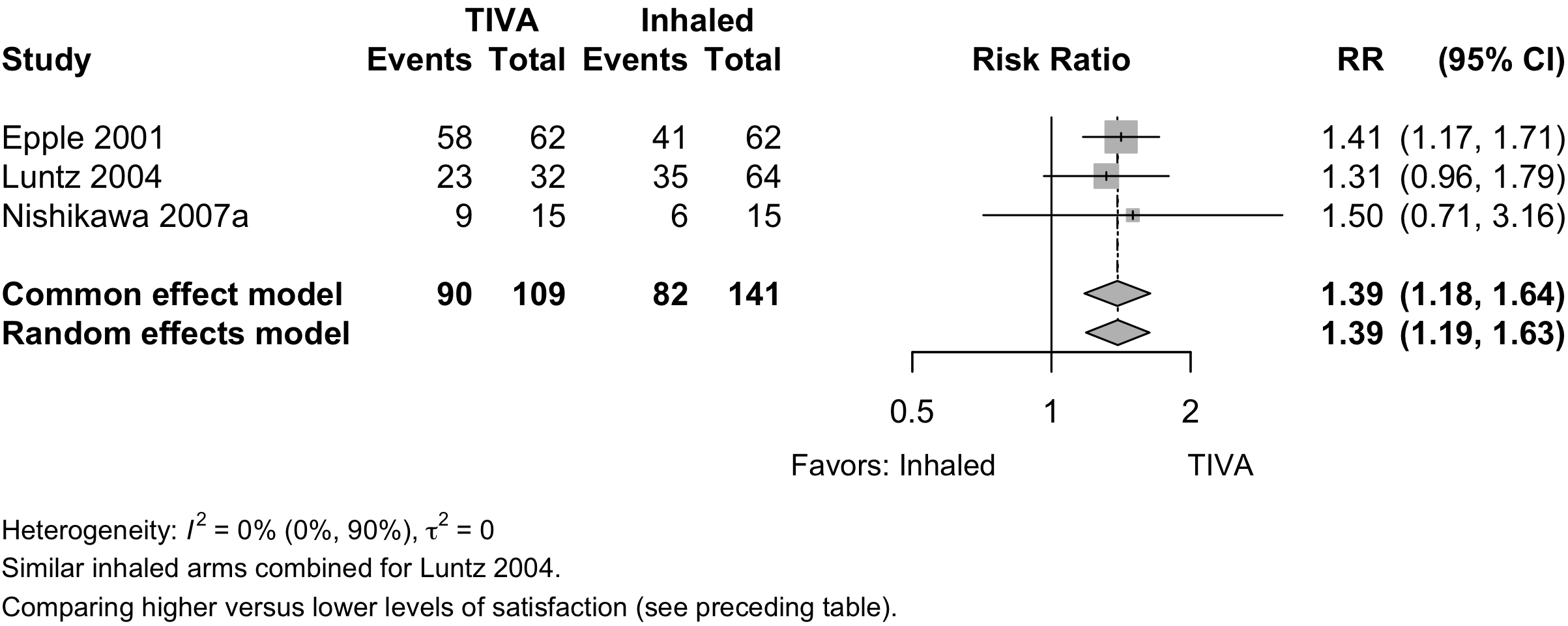
Length of Stay
| Study | N | Anesth | PSa | Ageb | LOSb | 0 – 35 days | Country |
|---|---|---|---|---|---|---|---|
| Randomized Clinical Trial - Cardiac | |||||||
| 48 | Inhaled | 34 | 64.5 (9.4) |
13.8 (4.6) |
South Korea | ||
| 47 | TIVA | 34 | 66.0 (7.3) |
12.6 (3.3) |
|||
| Randomized Clinical Trial - Thoracic | |||||||
| 190 | Inhaled | 123 | 72.4 (5.6) |
8.0 {6-11} |
China | ||
| 189 | TIVA | 123 | 72.8 (5.5) |
9.0 {6-13} |
|||
| Randomized Clinical Trial - Gastrointestinal/Abdominal | |||||||
| 153 | Inhaled | 123 | 66.0 [19-93] |
8.0 [2-48] |
Norway | ||
| 154 | TIVA | 123 | 65.0 [23-89] |
5.0 [2-50] |
|||
| 272 | Inhaled | NR | 65.0 {62-69} |
14.0 {11-16} |
China | ||
| 272 | TIVA | NR | 64.0 {62-68} |
15.0 {12-17} |
|||
| Randomized Clinical Trial - Various | |||||||
| 81 | Inhaled | 234 | 72.0 (7.0) |
8.0 (9.9) |
China | ||
| 83 | TIVA | 234 | 73.0 (8.0) |
9.0 (10.8) |
|||
| 597 | Inhaled | 123 | 71.0 [65-88] |
10.0 {7-14} |
China | ||
| 598 | TIVA | 123 | 72.0 [65-88] |
10.0 {7-14} |
|||
| Retrospective Cohort - Cardiac | |||||||
| 32 | Inhaled | NR | 78.3 (9.0) |
5.9 (3.3) |
USA | ||
| 84 | TIVA | NR | 79.6 (8.7) |
3.8 (3.3) |
|||
| Retrospective Cohort - Orthopedic | |||||||
| 5,140 | Inhaled | NR | 74.4 (7.4) |
31.4 (14.4) |
Japan | ||
| 5,140 | TIVA | NR | 74.5 (7.2) |
32.5 (18.4) |
|||
| 26 | Inhaled | 4 | 85.6 (7.8) |
14.5 (17.8) |
Taiwan | ||
| 20 | TIVA | 4 | 83.8 (9.1) |
8.8 (3.8) |
|||
| Retrospective Cohort - Various | |||||||
| 427,695 | Inhaled | NR | 76.3 |
21.0 (21.0) |
Japan | ||
| 142,565 | TIVA | NR | 76.6 |
21.0 (21.8) |
|||
| NR: not reported | |||||||
| a ASA Physical Status. | |||||||
| b Mean Med (SD)[Range]{IQR}. | |||||||
Pooled

Discharge Location
| Study | N | Arm | Agea | Country | Discharge to Institution | RR (95% CI) | |
|---|---|---|---|---|---|---|---|
| N (%) | 0 — 100% | ||||||
| Retrospective Cohort — Orthopedic | |||||||
| 26 | Inhaled | 85.6 (7.8) |
Taiwan | 8 (30.8) | |||
| 20 | TIVA | 83.8 (9.1) |
9 (45.0) | 1.46 (0.69-3.11) | |||
| Gen: general; Neur: neuraxial; RR: risk ratio. | |||||||
| a Mean Med (SD)[Range]{IQR}. | |||||||
Mortality
Table 17. Reported in-hospital, 30-day, and 1-year mortality in randomized clinical trials.
| Study | N | Arm | Surgery | ASA | Agea | Mortality | RD (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| PS | N (%) | 0 - 100% | ||||||
| Hospital | ||||||||
| 81 | Inhaled | Various | 234 | 72.0 (7.0) |
2 (2.5) | — | ||
| 83 | TIVA | 73.0 (8.0) |
1 (1.2) | -1.3% (-5.4, 2.9) | ||||
| 30-day | ||||||||
| 44 | Inhaled | Various | 1234 | 65.0 (11.8) |
0 (0) | — | ||
| 44 | TIVA | 64.0 (12.2) |
1 (2.3) | 2.3% (-3.8, 8.3) | ||||
| 97 | Inhaled | Vasc | 234 | 69.0 (9.0) |
4 (4.1) | — | ||
| 96 | TIVA | 67.0 (9.0) |
4 (4.2) | 0.0% (-5.6, 5.7) | ||||
| 153 | Inhaled | GI/Abd | 123 | 66.0 [19-93] |
0 (0) | — | ||
| 154 | TIVA | 65.0 [23-89] |
3 (1.9) | 1.9% (-0.6, 4.5) | ||||
| 190 | Inhaled | Thoracic | 123 | 72.4 (5.6) |
0 (0) | — | ||
| 189 | TIVA | 72.8 (5.5) |
0 (0) | 0.0% (-1.0, 1.0) | ||||
| 81 | Inhaled | Various | 234 | 72.0 (7.0) |
4 (4.9) | — | ||
| 83 | TIVA | 73.0 (8.0) |
3 (3.6) | -1.3% (-7.5, 4.9) | ||||
| 1-year | ||||||||
| 44 | Inhaled | Various | 1234 | 65.0 (11.8) |
1 (2.3) | — | ||
| 44 | TIVA | 64.0 (12.2) |
2 (4.5) | 2.3% (-5.3, 9.8) | ||||
| ASA PS: American Society of Anesthesiologists Physical Status; Vasc: vascular; GI/Abd: gastrointestinal/abdominal; RD: risk difference; NR: not reported. | ||||||||
| a Mean Med (SD)[Range]{IQR}. | ||||||||
Table 18. Reported in-hospital and 30-day mortality in nonrandomized designs (all retrospective cohort studies).
| Study | N | Arm | Surgery | ASA | Agea | Mortality | RD OR (95% CI)b | |
|---|---|---|---|---|---|---|---|---|
| PS | N (%) | 0 - 100% | ||||||
| Hospital | ||||||||
| 5,325 | Inhaled | Cardiac | NR | 64.9 |
116 (2.2) | — | ||
| 5,210 | TIVA | 64.7 |
172 (3.3) | 1.1% (0.5, 1.7) | ||||
| 386 | Inhaled | Various | 1234 | 65.5 (14.8) |
52 (13.5) | — | ||
| 100 | TIVA | 65.6 (14.9) |
22 (22.0) | 1.78 (1.08—2.92) | ||||
| 427,695 | Inhaled | Various | NR | 76.3 |
4,936 (1.2) | — | ||
| 142,565 | TIVA | 76.6 |
1,665 (1.2) | 1.01 (0.96—1.07) | ||||
| 30-day | ||||||||
| 5,325 | Inhaled | Cardiac | NR | 64.9 |
151 (2.8) | 0.7% (0.1, 1.3) | ||
| 5,210 | TIVA | 64.7 |
172 (3.3) | 1.1% (0.5, 1.7) | ||||
| 32 | Inhaled | Cardiac | NR | 78.3 (9.0) |
2 (6.2) | — | ||
| 84 | TIVA | 79.6 (8.7) |
0 (0) | -6.2% (-15.4, 2.9) | ||||
| 386 | Inhaled | Various | 1234 | 65.5 (14.8) |
35 (9.1) | — | ||
| 100 | TIVA | 65.6 (14.9) |
17 (17.0) | 2.6 (1.14—5.93) | ||||
| 1,202 | Inhaled | Various | 34 | 62.7 (13.7) |
51 (4.2) | — | ||
| 1,374 | TIVA | 65.6 (12.8) |
22 (1.6) | -2.6% (-4.0, -1.3) | ||||
| 8,722 | Inhaled | GI/Abd | 1234 | 71.0 {63-78} |
278 (3.2) | — | ||
| 8,722 | TIVA | 71.0 {64-77} |
280 (3.2) | 0.99 (0.84—1.18) | ||||
| 26 | Inhaled | Ortho | 4 | 85.6 (7.8) |
1 (3.8) | — | ||
| 20 | TIVA | 83.8 (9.1) |
1 (5.0) | 1.2% (-10.9, 13.2) | ||||
| 1-year | ||||||||
| 26 | Inhaled | Ortho | 4 | 85.6 (7.8) |
3 (11.5) | 7.7% (-6.6, 22.0) | ||
| 20 | TIVA | 83.8 (9.1) |
3 (15.0) | 11.2% (-6.2, 28.5) | ||||
| ASA PS: American Society of Anesthesiologists Physical Status; RD: risk difference; GI: gastrointestinal; Abd: abdominal (includes hepatic); Various: more that one procedure category. | ||||||||
| a Mean Med (SD)[Range]{IQR}. | ||||||||
| b Odds ratios for studies reporting adjusted results (e.g., propensity matched). | ||||||||
Pooled

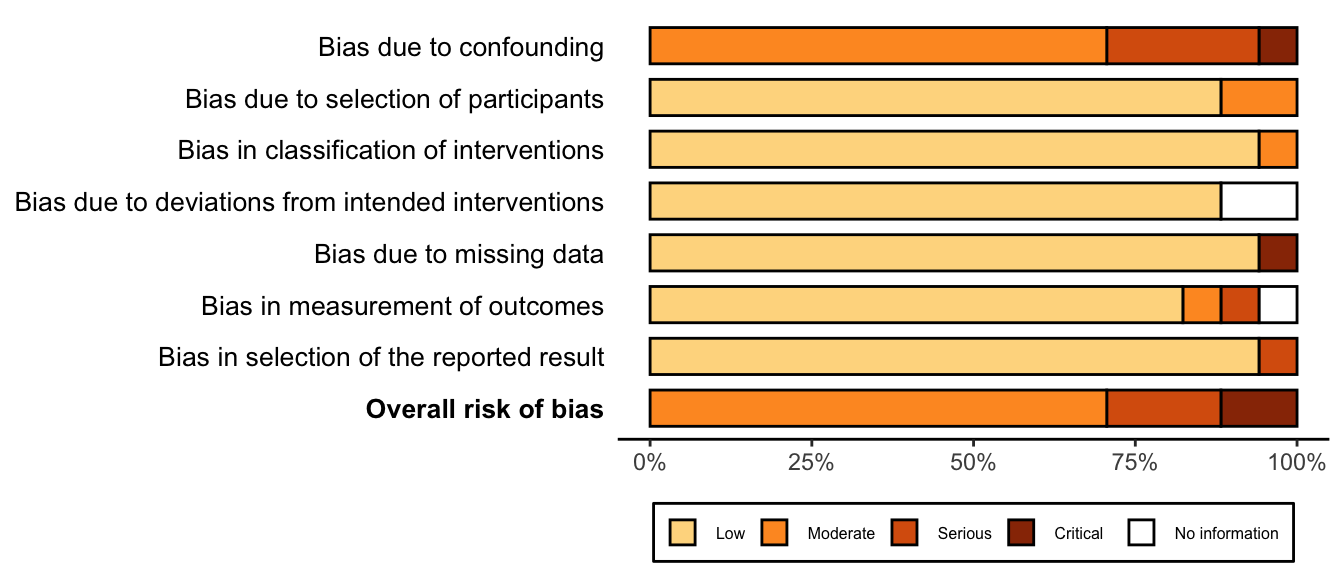
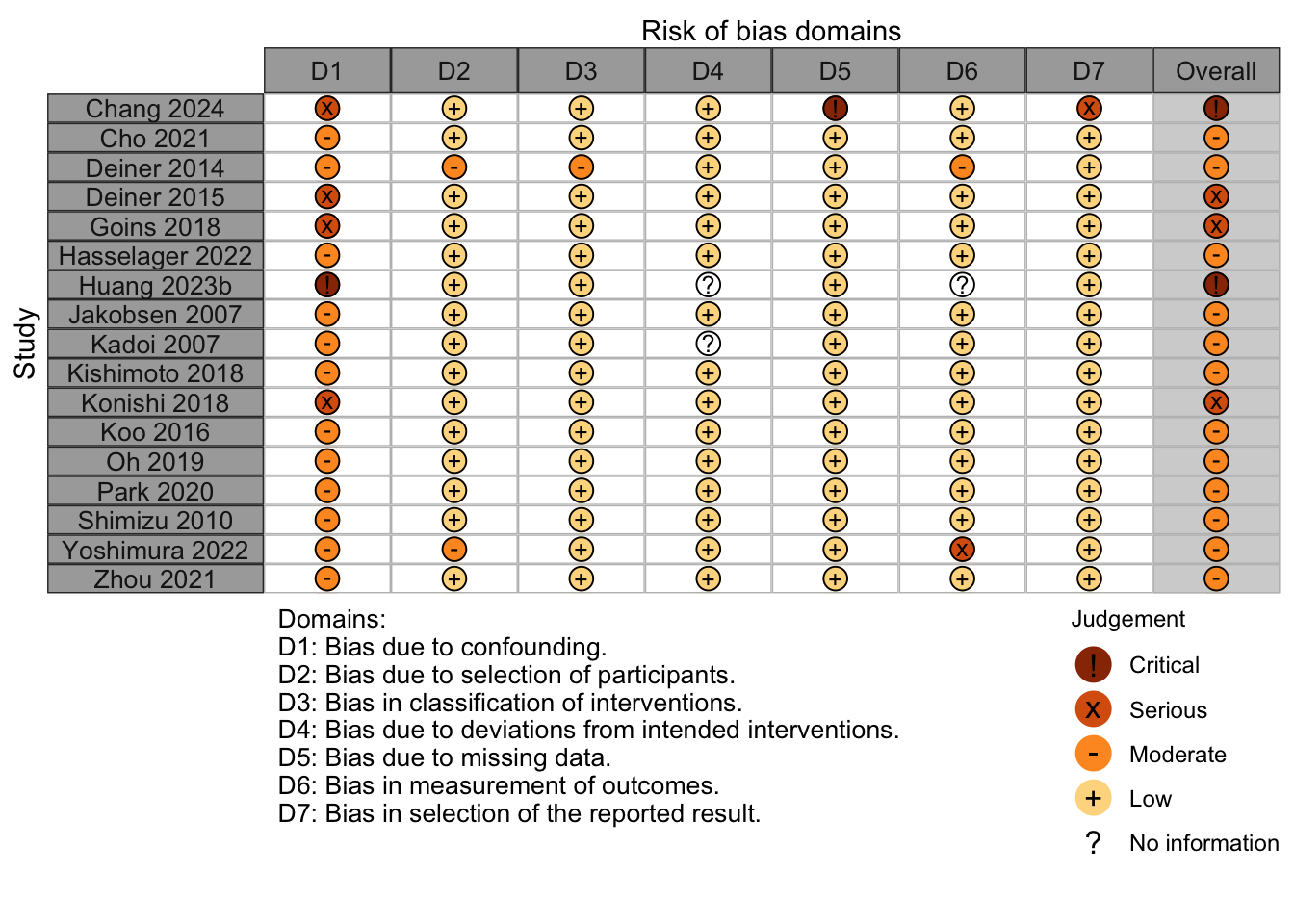
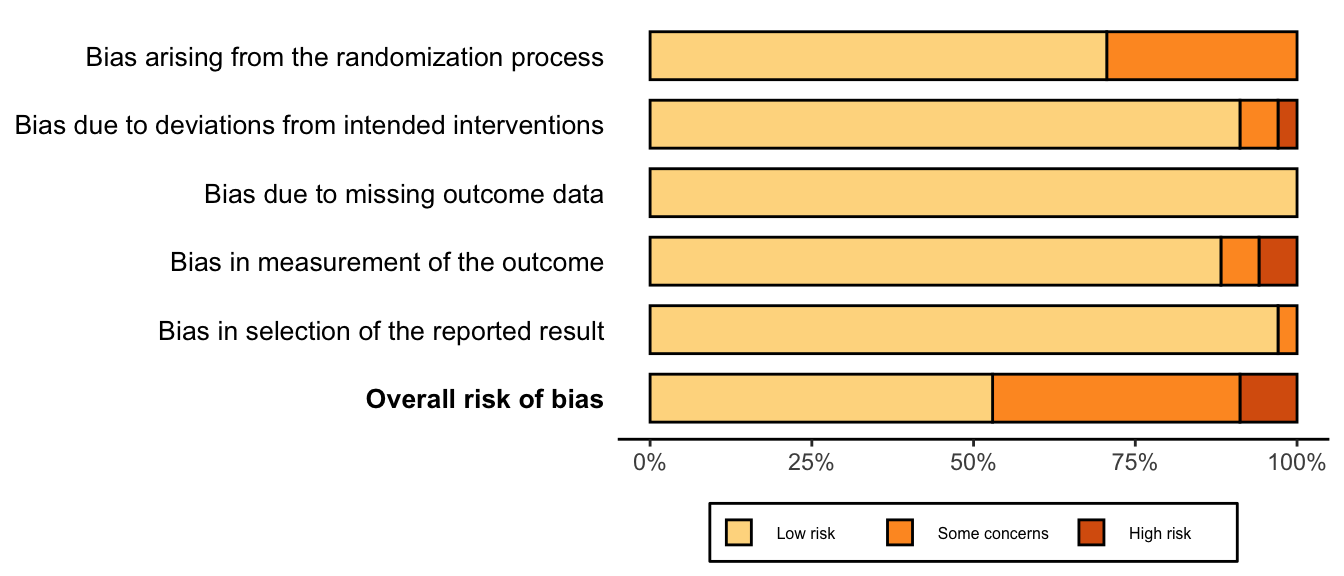
Risk of Bias
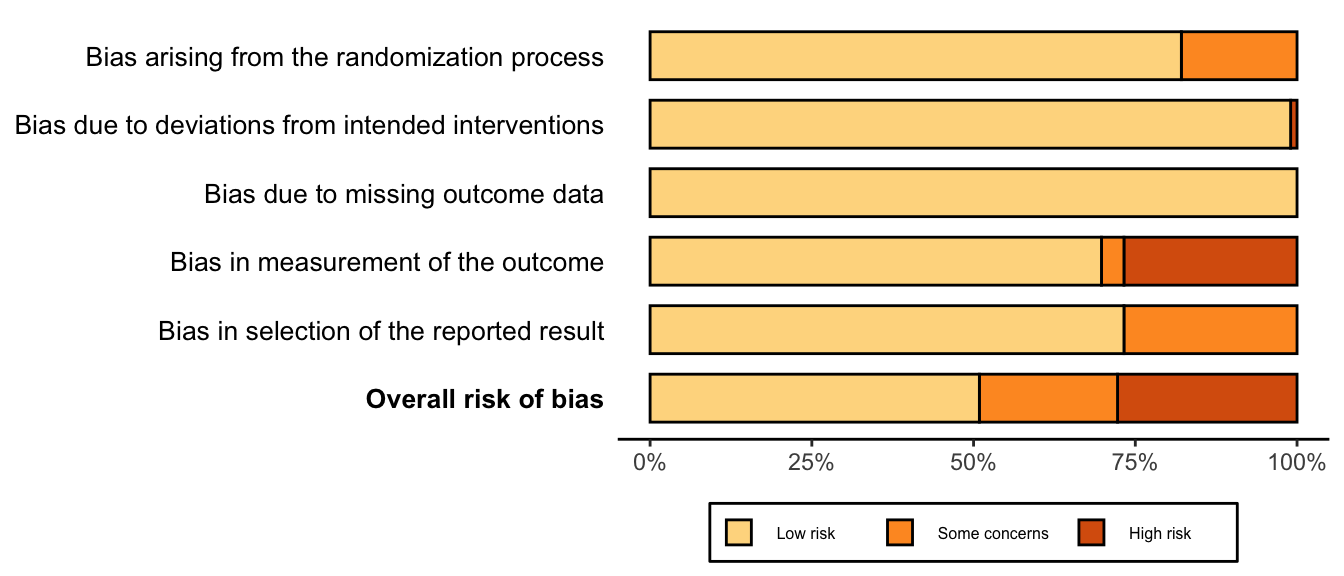
Randomized
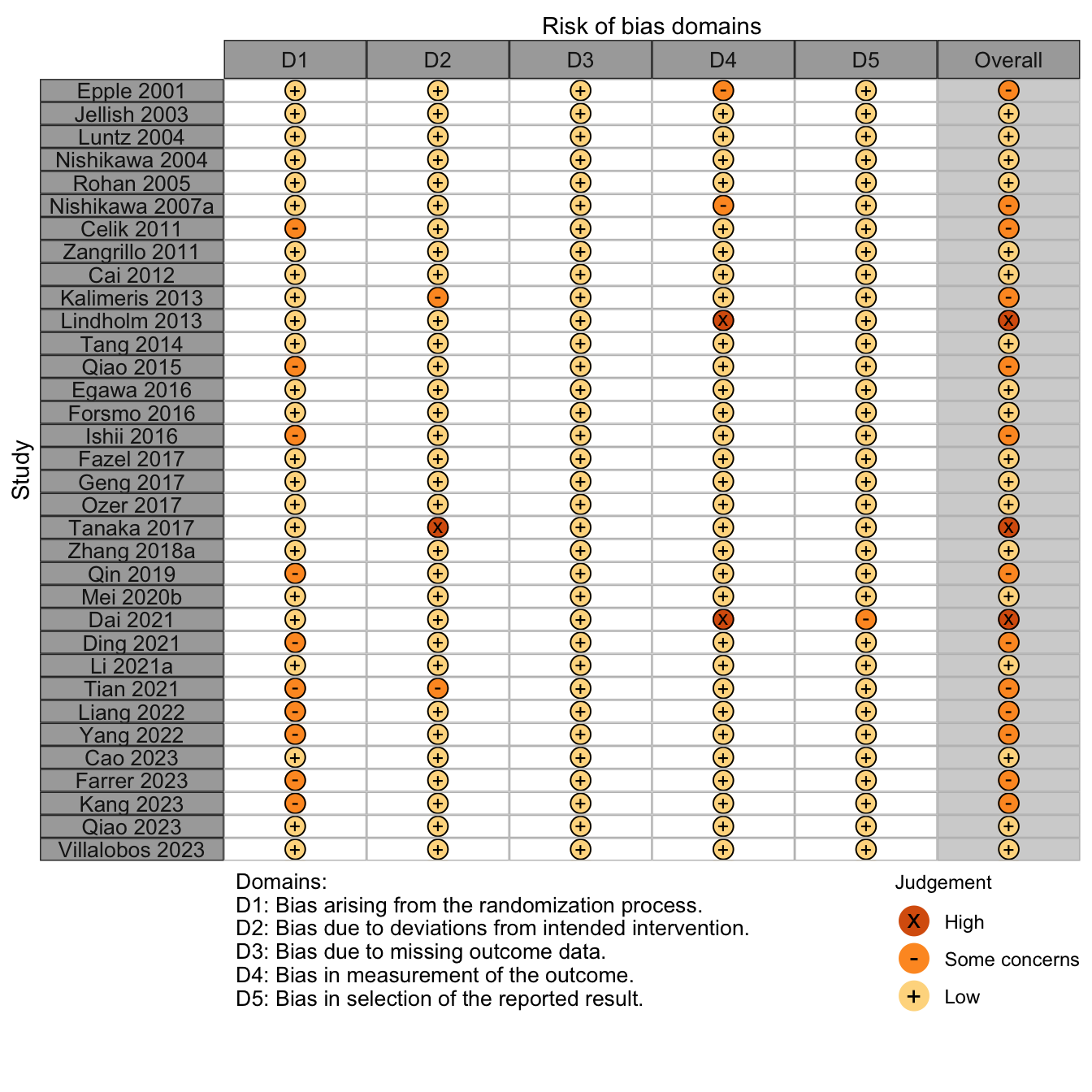
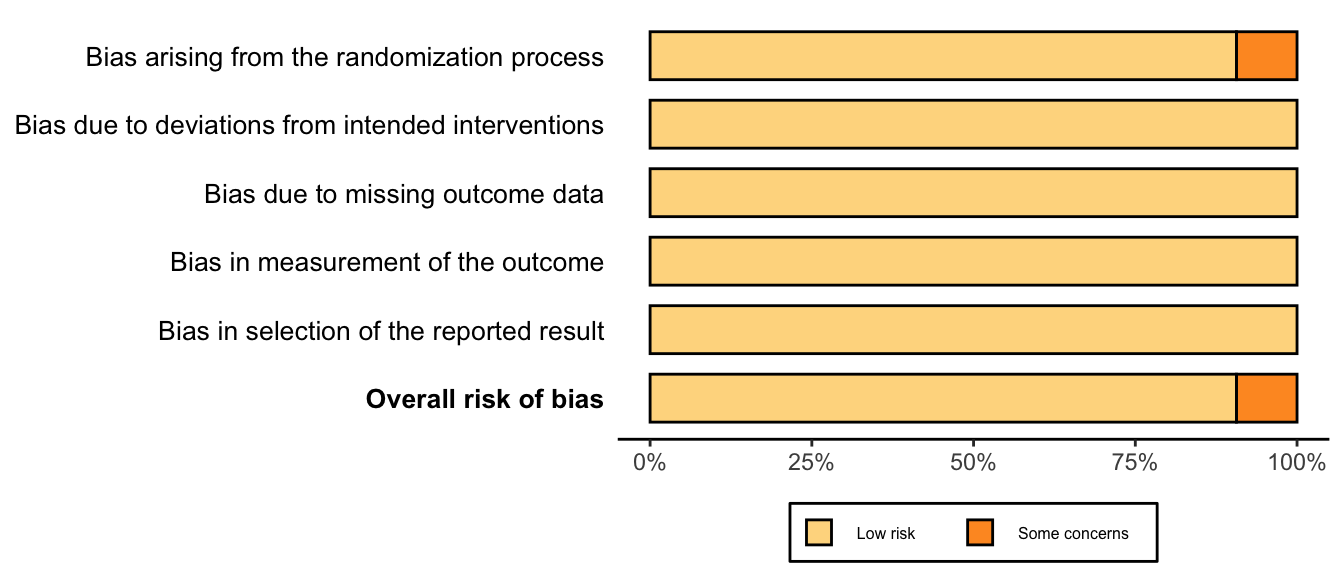
Nonrandomized